A comprehensive analysis of the effect of DSP4 on the locus coeruleus noradrenergic system in the rat
- PMID: 20045445
- PMCID: PMC4060967
- DOI: 10.1016/j.neuroscience.2009.12.027
A comprehensive analysis of the effect of DSP4 on the locus coeruleus noradrenergic system in the rat
Abstract
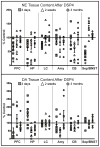
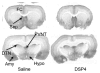
Degeneration of the noradrenergic neurons in the locus coeruleus (LC) is a major component of Alzheimer's (AD) and Parkinson's disease (PD), but the consequence of noradrenergic neuronal loss has different effects on the surviving neurons in the two disorders. Therefore, understanding the consequence of noradrenergic neuronal loss is important in determining the role of this neurotransmitter in these neurodegenerative disorders. The goal of the study was to determine if the neurotoxin N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP4) could be used as a model for either (or both) AD or PD. Rats were administered DSP4 and sacrificed 3 days 2 weeks and 3 months later. DSP4-treatment resulted in a rapid, though transient reduction in norepinephrine (NE) and NE transporter (NET) in many brain regions receiving variable innervation from the LC. Alpha(1)-adrenoreceptors binding site concentrations were unchanged in all brain regions at all three time points. However, an increase in alpha(2)-AR was observed in many different brain regions 2 weeks and 3 months after DSP4. These changes observed in forebrain regions occurred without a loss in LC noradrenergic neurons. Expression of synthesizing enzymes or NET did not change in amount of expression/neuron despite the reduction in NE tissue content and NET binding site concentrations at early time points, suggesting no compensatory response. In addition, DSP4 did not affect basal activity of LC at any time point in anesthetized animals, but 2 weeks after DSP4 there is a significant increase in irregular firing of noradrenergic neurons. These data indicate that DSP4 is not a selective LC noradrenergic neurotoxin, but does affect noradrenergic neuron terminals locally, as evident by the changes in transmitter and markers at terminal regions. However, since DSP4 did not result in a loss of noradrenergic neurons, it is not considered an adequate model for noradrenergic neuronal loss observed in AD and PD.
Published by Elsevier Ltd.
Figures

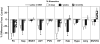


Similar articles
-
DSP4, a selective neurotoxin for the locus coeruleus noradrenergic system. A review of its mode of action.Neurotox Res. 2015 Jan;27(1):15-30. doi: 10.1007/s12640-014-9482-z. Epub 2014 Jun 26. Neurotox Res. 2015. PMID: 24964753 Review.
-
Noradrenergic depletion potentiates beta -amyloid-induced cortical inflammation: implications for Alzheimer's disease.J Neurosci. 2002 Apr 1;22(7):2434-42. doi: 10.1523/JNEUROSCI.22-07-02434.2002. J Neurosci. 2002. PMID: 11923407 Free PMC article.
-
Potentiation of parkinsonian symptoms by depletion of locus coeruleus noradrenaline in 6-hydroxydopamine-induced partial degeneration of substantia nigra in rats.Eur J Neurosci. 2003 Jun;17(12):2586-92. doi: 10.1046/j.1460-9568.2003.02684.x. Eur J Neurosci. 2003. PMID: 12823465
-
A noradrenergic lesion aggravates the effects of systemic inflammation on the hippocampus of aged rats.PLoS One. 2017 Dec 19;12(12):e0189821. doi: 10.1371/journal.pone.0189821. eCollection 2017. PLoS One. 2017. PMID: 29261743 Free PMC article.
-
Selective Lifelong Destruction of Brain Monoaminergic Nerves Through Perinatal DSP-4 Treatment.Curr Top Behav Neurosci. 2016;29:51-71. doi: 10.1007/7854_2015_398. Curr Top Behav Neurosci. 2016. PMID: 26427851 Review.
Cited by
-
Effects of noradrenergic denervation on L-DOPA-induced dyskinesia and its treatment by α- and β-adrenergic receptor antagonists in hemiparkinsonian rats.Pharmacol Biochem Behav. 2012 Jan;100(3):607-15. doi: 10.1016/j.pbb.2011.09.009. Epub 2011 Sep 25. Pharmacol Biochem Behav. 2012. PMID: 21978941 Free PMC article.
-
Locus Coeruleus Degeneration Induces Forebrain Vascular Pathology in a Transgenic Rat Model of Alzheimer's Disease.J Alzheimers Dis. 2019;70(2):371-388. doi: 10.3233/JAD-190090. J Alzheimers Dis. 2019. PMID: 31177220 Free PMC article.
-
Evidence for a specialized role of the locus coeruleus noradrenergic system in cortical circuitries and behavioral operations.Brain Res. 2016 Jun 15;1641(Pt B):197-206. doi: 10.1016/j.brainres.2015.11.022. Epub 2015 Nov 25. Brain Res. 2016. PMID: 26607255 Free PMC article. Review.
-
Noradrenergic Modulation of Cognition in Health and Disease.Neural Plast. 2017;2017:6031478. doi: 10.1155/2017/6031478. Epub 2017 May 3. Neural Plast. 2017. PMID: 28596922 Free PMC article. Review.
-
Noradrenergic pathway from the locus coeruleus to heart is implicated in modulating SUDEP.iScience. 2023 Feb 27;26(4):106284. doi: 10.1016/j.isci.2023.106284. eCollection 2023 Apr 21. iScience. 2023. PMID: 36968083 Free PMC article.
References
-
- Archer T, Fredriksson A. Influence of noradrenaline denervation on MPTP-induced defcitis in mice. J Neural Transm. 2006;113:1119–1129. - PubMed
-
- Bertrand E, Lechowicz W, Szpak GM, Dynecki J. Qualitative and quantitative analysis of locus coeruleus neurons in Parkinson's disease. Folia Neuropathaol. 1997;35:80–86. - PubMed
-
- Bondareff W, Mountjoy CQ, Roth M. Selective loss of neurons of origin of adrenergic projection to cerebral cortex (nucleus locus coeruleus) in senile dementia. Lancet. 1981;1:783–784. - PubMed
-
- Booze RM, Hall JA, Cress NM, Miller GD, Davis JN. DESP-4 treatment produces abnormal tyrosine hydroxylase immunoreactive fibers in rat hippocampus. Exp Neurol. 1988;101:75–86. - PubMed
-
- Cash R, Dennis T, L'Heureux R, Raisman R, Javoy-Agid F, Scatton B. Parkinson's disease and dementia: norepinephrine and dopamine in locus coeruleus. Neurology. 1987;37:42–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

