Matrix metalloproteinase-dependent shedding of intercellular adhesion molecule-5 occurs with long-term potentiation
- PMID: 20045450
- PMCID: PMC3535483
- DOI: 10.1016/j.neuroscience.2009.12.061
Matrix metalloproteinase-dependent shedding of intercellular adhesion molecule-5 occurs with long-term potentiation
Abstract
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases that can be released or activated in a neuronal activity dependent manner. Although pathologically elevated levels of MMPs may be synaptotoxic, physiologically appropriate levels of MMPs may instead enhance synaptic transmission. MMP inhibitors can block long term potentiation (LTP), and at least one family member can affect an increase in the volume of dendritic spines. While the mechanism by which MMPs affect these changes is not completely understood, one possibility is that the cleavage of specific synaptic cell adhesion molecules plays a role. In the present study, we have examined the ability of neuronal activity to stimulate rapid MMP dependent shedding of the intercellular adhesion molecule-5 (ICAM-5), a synaptic adhesion molecule that is thought to inhibit the maturation and enlargement of dendritic spines. Since such cleavage would likely occur within minutes if it were relevant to a process such as LTP, we focused on post stimulus time points of 30 min or less. We show that NMDA can stimulate rapid shedding of ICAM-5 from cortical neurons in dissociated cell cultures and that such shedding is diminished by pretreatment of cultures with inhibitors that target MMP-3 and -9, proteases thought to influence synaptic plasticity. Additional studies suggest that MMP mediated cleavage of ICAM-5 occurs at amino acid 780, so that the major portion of the ectodomain is released. Since reductions in ICAM-5 have been linked to changes in dendritic spine morphology that are associated with LTP, we also examined the possibility that MMP dependent ICAM-5 shedding occurs following high frequency tetanic stimulation of murine hippocampal slices. Results show that the shedding of ICAM-5 occurs in association with LTP, and that both LTP and the associated ICAM-5 shedding are reduced when slices are pretreated with an MMP inhibitor. Together, these findings suggest that neuronal activity is linked to the shedding of a molecule that may inhibit dendritic spine enlargement and that MMPs can affect this change. While further studies will be necessary to determine the extent to which cleavage of ICAM-5 in particular contributes to MMP dependent LTP, our data support an emerging body of literature suggesting that MMPs are critical mediators of synaptic plasticity.
Copyright (c) 2010 IBRO. All rights reserved.
Figures
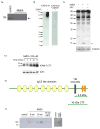
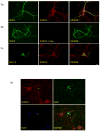

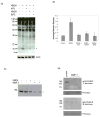

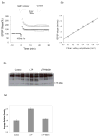
Similar articles
-
Soluble ICAM-5, a product of activity dependent proteolysis, increases mEPSC frequency and dendritic expression of GluA1.PLoS One. 2013 Jul 2;8(7):e69136. doi: 10.1371/journal.pone.0069136. Print 2013. PLoS One. 2013. PMID: 23844251 Free PMC article.
-
MMPs and soluble ICAM-5 increase neuronal excitability within in vitro networks of hippocampal neurons.PLoS One. 2012;7(8):e42631. doi: 10.1371/journal.pone.0042631. Epub 2012 Aug 13. PLoS One. 2012. PMID: 22912716 Free PMC article.
-
Methamphetamine-associated cleavage of the synaptic adhesion molecule intercellular adhesion molecule-5.J Neurochem. 2011 Aug;118(4):521-32. doi: 10.1111/j.1471-4159.2010.07153.x. Epub 2011 Jan 19. J Neurochem. 2011. PMID: 21166806 Free PMC article.
-
Long-term potentiation in cultured hippocampal neurons.Semin Cell Dev Biol. 2011 Jul;22(5):506-13. doi: 10.1016/j.semcdb.2011.07.017. Epub 2011 Jul 22. Semin Cell Dev Biol. 2011. PMID: 21807105 Review.
-
Regulation of neuronal PKA signaling through AKAP targeting dynamics.Eur J Cell Biol. 2006 Jul;85(7):627-33. doi: 10.1016/j.ejcb.2006.01.010. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16504338 Review.
Cited by
-
Copper signaling in the mammalian nervous system: synaptic effects.J Neurosci Res. 2013 Jan;91(1):2-19. doi: 10.1002/jnr.23143. Epub 2012 Nov 1. J Neurosci Res. 2013. PMID: 23115049 Free PMC article. Review.
-
Plasticity of Spine Structure: Local Signaling, Translation and Cytoskeletal Reorganization.Front Synaptic Neurosci. 2018 Aug 29;10:29. doi: 10.3389/fnsyn.2018.00029. eCollection 2018. Front Synaptic Neurosci. 2018. PMID: 30210329 Free PMC article. Review.
-
Matrix metalloproteinase signals following neurotrauma are right on cue.Cell Mol Life Sci. 2019 Aug;76(16):3141-3156. doi: 10.1007/s00018-019-03176-4. Epub 2019 Jun 6. Cell Mol Life Sci. 2019. PMID: 31168660 Free PMC article. Review.
-
Long-term depression-inducing stimuli promote cleavage of the synaptic adhesion molecule NGL-3 through NMDA receptors, matrix metalloproteinases and presenilin/γ-secretase.Philos Trans R Soc Lond B Biol Sci. 2013 Dec 2;369(1633):20130158. doi: 10.1098/rstb.2013.0158. Print 2014 Jan 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24298159 Free PMC article.
-
Disruption of perineuronal nets increases the frequency of sharp wave ripple events.Hippocampus. 2018 Jan;28(1):42-52. doi: 10.1002/hipo.22804. Epub 2017 Sep 26. Hippocampus. 2018. PMID: 28921856 Free PMC article.
References
-
- Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin) J Biol Chem. 2001;276:28261–28267. - PubMed
-
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. - PubMed
-
- Arendt Y, Banci L, Bertini I, Cantini F, Cozzi R, Del Conte R, Gonnelli L. Catalytic domain of MMP20 (Enamelysin) - the NMR structure of a new matrix metalloproteinase. FEBS Lett. 2007;581:4723–4726. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

