UDP-GlC:glycoprotein glucosyltransferase-glucosidase II, the ying-yang of the ER quality control
- PMID: 20045480
- PMCID: PMC2883647
- DOI: 10.1016/j.semcdb.2009.12.014
UDP-GlC:glycoprotein glucosyltransferase-glucosidase II, the ying-yang of the ER quality control
Abstract
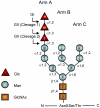
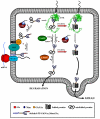
The N-glycan-dependent quality control of glycoprotein folding prevents endoplasmic to Golgi exit of folding intermediates, irreparably misfolded glycoproteins and incompletely assembled multimeric complexes. It also enhances folding efficiency by preventing aggregation and facilitating formation of proper disulfide bonds. The control mechanism essentially involves four components, resident lectin-chaperones that recognize monoglucosylated polymannose glycans, a lectin-associated oxidoreductase acting on monoglucosylated glycoproteins, a glucosyltransferase that creates monoglucosytlated epitopes in protein-linked glycans and a glucosidase that removes the glucose units added by the glucosyltransferase. This last enzyme is the only mechanism component sensing glycoprotein conformations as it creates monoglucosylated glycans exclusively in not properly folded species or in not completely assembled complexes. The glucosidase is a dimeric heterodimer composed of a catalytic subunit and an additional one that is partially responsible for the ER localization of the enzyme and for the enhancement of the deglucosylation rate as its mannose 6-phosphate receptor homologous domain presents the substrate to the catalytic site. This review deals with our present knowledge on the glucosyltransferase and the glucosidase.
Figures
Similar articles
-
How sugars convey information on protein conformation in the endoplasmic reticulum.Semin Cell Dev Biol. 2007 Dec;18(6):732-42. doi: 10.1016/j.semcdb.2007.09.006. Epub 2007 Sep 8. Semin Cell Dev Biol. 2007. PMID: 17997334 Free PMC article. Review.
-
A sweet code for glycoprotein folding.FEBS Lett. 2015 Nov 14;589(22):3379-87. doi: 10.1016/j.febslet.2015.07.021. Epub 2015 Jul 28. FEBS Lett. 2015. PMID: 26226420 Review.
-
Role of N-oligosaccharide endoplasmic reticulum processing reactions in glycoprotein folding and degradation.Biochem J. 2000 May 15;348 Pt 1(Pt 1):1-13. Biochem J. 2000. PMID: 10794707 Free PMC article. Review.
-
Glucosidase II and N-glycan mannose content regulate the half-lives of monoglucosylated species in vivo.Mol Biol Cell. 2011 Jun 1;22(11):1810-23. doi: 10.1091/mbc.E11-01-0019. Epub 2011 Apr 6. Mol Biol Cell. 2011. PMID: 21471007 Free PMC article.
-
Role of N-linked polymannose oligosaccharides in targeting glycoproteins for endoplasmic reticulum-associated degradation.Cell Mol Life Sci. 2004 May;61(9):1025-41. doi: 10.1007/s00018-004-4037-8. Cell Mol Life Sci. 2004. PMID: 15112051 Free PMC article. Review.
Cited by
-
Htm1p-Pdi1p is a folding-sensitive mannosidase that marks N-glycoproteins for ER-associated protein degradation.Proc Natl Acad Sci U S A. 2016 Jul 12;113(28):E4015-24. doi: 10.1073/pnas.1608795113. Epub 2016 Jun 28. Proc Natl Acad Sci U S A. 2016. PMID: 27357682 Free PMC article.
-
Protein Aggregation in the ER: Calm behind the Storm.Cells. 2021 Nov 28;10(12):3337. doi: 10.3390/cells10123337. Cells. 2021. PMID: 34943844 Free PMC article. Review.
-
Revisiting CFTR Interactions: Old Partners and New Players.Int J Mol Sci. 2021 Dec 7;22(24):13196. doi: 10.3390/ijms222413196. Int J Mol Sci. 2021. PMID: 34947992 Free PMC article. Review.
-
Structural and functional comparison of SARS-CoV-2-spike receptor binding domain produced in Pichia pastoris and mammalian cells.Sci Rep. 2020 Dec 11;10(1):21779. doi: 10.1038/s41598-020-78711-6. Sci Rep. 2020. PMID: 33311634 Free PMC article.
-
The Crucial Role of Demannosylating Asparagine-Linked Glycans in ERADicating Misfolded Glycoproteins in the Endoplasmic Reticulum.Front Plant Sci. 2021 Jan 12;11:625033. doi: 10.3389/fpls.2020.625033. eCollection 2020. Front Plant Sci. 2021. PMID: 33510762 Free PMC article. Review.
References
-
- Parodi AJ, Cazzulo JJ. Protein glycosylation in Trypanosoma cruzi. II. Partial characterization of protein-bound oligosaccharides labeled “in vivo”. J Biol Chem. 1982;257:7641–5. - PubMed
-
- Trombetta SE, Parodi AJ. Purification to apparent homogeneity and partial characterization of rat liver UDP-glucose:glycoprotein glucosyltransferase. J Biol Chem. 1992;267:9236–40. - PubMed
-
- Ware FE, Vassilakos A, Peterson PA, Jackson MR, Lehrman MA, Williams DB. The molecular chaperone calnexin binds Glc1Man9GlcNAc2 oligosaccharide as an initial step in recognizing unfolded glycoproteins. J Biol Chem. 1995;270:4697–704. - PubMed
-
- Babour A, Beckerich JM, Gaillardin C. Identification of an UDP-Glc:glycoprotein glucosyltransferase in the yeast Yarrowia lipolytica. Yeast. 2004;21:11–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

