Polar 3-alkylidene-5-pivaloyloxymethyl-5'-hydroxymethyl-gamma-lactones as protein kinase C ligands and antitumor agents
- PMID: 20045644
- PMCID: PMC3725291
- DOI: 10.1016/j.bmcl.2009.12.058
Polar 3-alkylidene-5-pivaloyloxymethyl-5'-hydroxymethyl-gamma-lactones as protein kinase C ligands and antitumor agents
Abstract
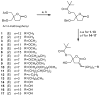
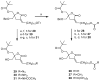
A series of DAG-lactones with polar 3-alkylidene substituents have been investigated as PKC-alpha ligands and antitumor agents. Extensive analysis of structure-activity relationships for the 3-alkylidene chain revealed that polar groups such as ether, hydroxyl, aldehyde, ester, acyloxy, and amido were tolerated with similar binding affinities and reduced lipophilicities compared to the corresponding unsubstituted alkylidene chain. Among the derivatives, compounds 5, 6 and 8 with an ether type of side chain showed high binding affinities in range of K(i)= 3-5 nM and excellent antitumor profiles, particularly against the colo205 colon cancer and the K562 leukemia cell lines.
Copyright (c) 2010 Elsevier Ltd. All rights reserved.
Figures
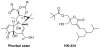
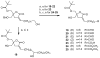
Similar articles
-
Conformationally constrained analogues of diacylglycerol (DAG). 16. How much structural complexity is necessary for recognition and high binding affinity to protein kinase C?J Med Chem. 2000 Mar 9;43(5):921-44. doi: 10.1021/jm9904607. J Med Chem. 2000. PMID: 10715158
-
Conformationally constrained analogues of diacylglycerol. 10. Ultrapotent protein kinase C ligands based on a racemic 5-disubstituted tetrahydro-2-furanone template.J Med Chem. 1996 Jan 5;39(1):19-28. doi: 10.1021/jm950276v. J Med Chem. 1996. PMID: 8568806
-
Design and synthesis of bioisosteres of ultrapotent protein kinase C (PKC) ligand, 5-acetoxymethyl-5-hydroxymethyl-3-alkylidene tetrahydro-2-furanone.Arch Pharm Res. 1998 Aug;21(4):452-7. doi: 10.1007/BF02974642. Arch Pharm Res. 1998. PMID: 9875475
-
The transition from a pharmacophore-guided approach to a receptor-guided approach in the design of potent protein kinase C ligands.Pharmacol Ther. 1999 May-Jun;82(2-3):251-61. doi: 10.1016/s0163-7258(98)00048-5. Pharmacol Ther. 1999. PMID: 10454202 Review.
-
Synthetic diacylglycerols (DAG) and DAG-lactones as activators of protein kinase C (PK-C).Acc Chem Res. 2003 Jun;36(6):434-43. doi: 10.1021/ar020124b. Acc Chem Res. 2003. PMID: 12809530 Review.
Cited by
-
Natural and Synthetic Lactones Possessing Antitumor Activities.Int J Mol Sci. 2021 Jan 21;22(3):1052. doi: 10.3390/ijms22031052. Int J Mol Sci. 2021. PMID: 33494352 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

