Distinct enhancers at the Pax3 locus can function redundantly to regulate neural tube and neural crest expressions
- PMID: 20045680
- PMCID: PMC2830354
- DOI: 10.1016/j.ydbio.2009.12.030
Distinct enhancers at the Pax3 locus can function redundantly to regulate neural tube and neural crest expressions
Abstract
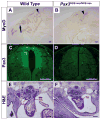
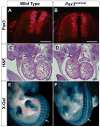
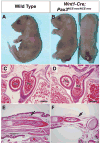
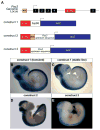
Pax3 is a transcription factor expressed in somitic mesoderm, dorsal neural tube and pre-migratory neural crest during embryonic development. We have previously identified cis-acting enhancer elements within the proximal upstream genomic region of Pax3 that are sufficient to direct functional expression of Pax3 in neural crest. These elements direct expression of a reporter gene to pre-migratory neural crest in transgenic mice, and transgenic expression of a Pax3 cDNA using these elements is sufficient to rescue neural crest development in mice otherwise lacking endogenous Pax3. We show here that deletion of these enhancer sequences by homologous recombination is insufficient to abrogate neural crest expression of Pax3 and results in viable mice. We identify a distinct enhancer in the fourth intron that is also capable of mediating neural crest expression in transgenic mice and zebrafish. Our analysis suggests the existence of functionally redundant neural crest enhancer modules for Pax3.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures


References
-
- Auerbach R. Analysis of the developmental effects of a lethal mutation in the house mouse. J Exper Zool. 1954;127:305–329.
-
- Bang AG, Papalopulu N, Goulding MD, Kintner C. Expression of Pax-3 in the lateral neural plate is dependent on a Wnt-mediated signal from posterior nonaxial mesoderm. Dev Biol. 1999;212:366–80. - PubMed
-
- Bang AG, Papalopulu N, Kintner C, Goulding MD. Expression of Pax-3 is initiated in the early neural plate by posteriorizing signals produced by the organizer and by posterior non-axial mesoderm. Development. 1997;124:2075–85. - PubMed
-
- Blackwood EM, Kadonaga JT. Going the distance: a current view of enhancer action. Science. 1998;281:60–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

