Mago Nashi, Tsunagi/Y14, and Ranshi form a complex that influences oocyte differentiation in Drosophila melanogaster
- PMID: 20045686
- PMCID: PMC2852135
- DOI: 10.1016/j.ydbio.2009.12.035
Mago Nashi, Tsunagi/Y14, and Ranshi form a complex that influences oocyte differentiation in Drosophila melanogaster
Abstract
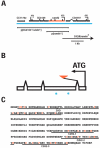
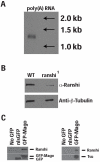
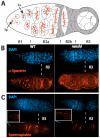
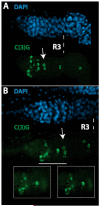
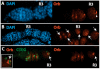
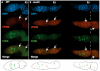
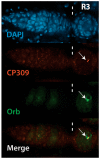
During Drosophila melanogaster oogenesis, a germline stem cell divides forming a cyst of 16 interconnected cells. One cell enters the oogenic pathway, and the remaining 15 differentiate as nurse cells. Although directed transport and localization of oocyte differentiation factors within the single cell are indispensible for selection, maintenance, and differentiation of the oocyte, the mechanisms regulating these events are poorly understood. Mago Nashi and Tsunagi/Y14, core components of the exon junction complex (a multiprotein complex assembled on spliced RNAs), are essential for restricting oocyte fate to a single cell and for localization of oskar mRNA. Here we provide evidence that Mago Nashi and Tsunagi/Y14 form an oogenic complex with Ranshi, a protein with a zinc finger-associated domain and zinc finger domains. Genetic analyses of ranshi reveal that (1) 16-cell cysts are formed, (2) two cells retain synaptonemal complexes, (3) all cells have endoreplicated DNA (as observed in nurse cells), and (4) oocyte-specific cytoplasmic markers accumulate and persist within a single cell but are not localized within the posterior pole of the presumptive oocyte. Our results indicate that Ranshi interacts with the exon junction complex to localize components essential for oocyte differentiation within the posterior pole of the presumptive oocyte.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures

References
-
- al-Mukhtar KA, Webb AC. An ultrastructural study of primordial germ cells, oogonia and early oocytes in Xenopus laevis. J. Embryol. Exp. Morphol. 1971;26:195–217. - PubMed
-
- Bolivar J, Huynh JR, Lopez-Schier H, Gonzalez C, St Johnston D, Gonzalez-Reyes A. Centrosome migration into the Drosophila oocyte is independent of BicD and egl, and of the organisation of the microtubule cytoskeleton. Development. 2001;128:1889–1897. - PubMed
-
- Brayer KJ, Segal DJ. Keep your fingers off my DNA: protein-protein interactions mediated by C2H2 zinc finger domains. Cell Biochem. Biophys. 2008;50:111–131. - PubMed
-
- Brown RS. Zinc finger proteins: getting a grip on RNA. Curr. Opin. Struct. Biol. 2005;15:94–98. - PubMed
-
- Büning J. The Insect Ovary: Ultrastructure, Previtellogenic Growth and Evolution. Chapman & Hall; New York: 1994.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

