Sepsis-induced myopathy
- PMID: 20046121
- PMCID: PMC3967515
- DOI: 10.1097/CCM.0b013e3181b6e439
Sepsis-induced myopathy
Abstract
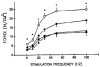
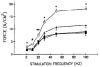
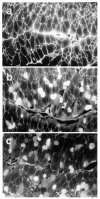
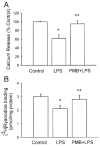
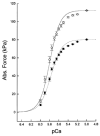
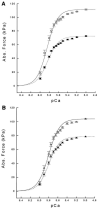
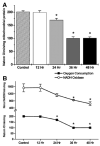
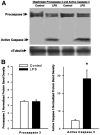
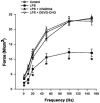
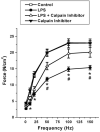
Sepsis is a major cause of morbidity and mortality in critically ill patients, and despite advances in management, mortality remains high. In survivors, sepsis increases the risk for the development of persistent acquired weakness syndromes affecting both the respiratory muscles and the limb muscles. This acquired weakness results in prolonged duration of mechanical ventilation, difficulty weaning, functional impairment, exercise limitation, and poor health-related quality of life. Abundant evidence indicates that sepsis induces a myopathy characterized by reductions in muscle force-generating capacity, atrophy (loss of muscle mass), and altered bioenergetics. Sepsis elicits derangements at multiple subcellular sites involved in excitation contraction coupling, such as decreasing membrane excitability, injuring sarcolemmal membranes, altering calcium homeostasis due to effects on the sarcoplasmic reticulum, and disrupting contractile protein interactions. Muscle wasting occurs later and results from increased proteolytic degradation as well as decreased protein synthesis. In addition, sepsis produces marked abnormalities in muscle mitochondrial functional capacity and when severe, these alterations correlate with increased death. The mechanisms leading to sepsis-induced changes in skeletal muscle are linked to excessive localized elaboration of proinflammatory cytokines, marked increases in free-radical generation, and activation of proteolytic pathways that are upstream of the proteasome including caspase and calpain. Emerging data suggest that targeted inhibition of these pathways may alter the evolution and progression of sepsis-induced myopathy and potentially reduce the occurrence of sepsis-mediated acquired weakness syndromes.
Figures

References
-
- De Jonghe B, Lacherade JC, Durand MC, et al. Critical illness neuromuscular syndromes. Neurol Clin. 2008;26:507–520. - PubMed
-
- Herridge MS, Batt J, Hopkins RO. The pathophysiology of long-term neuromuscular and cognitive outcomes following critical illness. Crit Care Clin. 2008;24:179–199. - PubMed
-
- Latronico N, Guarneri B. Critical illness myopathy and neuropathy. Minerva Anestesiol. 2008;74:319–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

