Light-Induced Toxic Effects of Tamoxifen: A Chemotherapeutic and Chemopreventive Agent
- PMID: 20046228
- PMCID: PMC2637528
- DOI: 10.1016/j.jphotochem.2008.09.013
Light-Induced Toxic Effects of Tamoxifen: A Chemotherapeutic and Chemopreventive Agent
Abstract
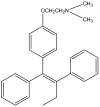
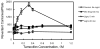
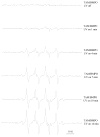
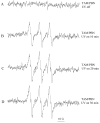
Tamoxifen is a powerful drug used to treat breast cancer patients, and more than 500,000 women in the U. S. are being treated with this drug. In our study, tamoxifen is found to be photomutagenic in Salmonella typhimurium TA102 at concentrations as low as 0.08 muM and reaches maximum photomutagenicity at 0.4 muM under a light dose equivalent to 20 min sunlight. These concentrations are comparable to the plasma tamoxifen concentration of 0.4 to 3 muM for patients undergoing tamoxifen therapy. The toxicity seems to be the result of DNA damage and/or lipid peroxidation caused by light irradiation of tamoxifen. The DNA damage caused by irradiation of PhiX174 DNA in the presence of tamoxifen appears to be formation of DNA-tamoxifen covalent adducts, not single strand/double strand cleavages, and there is no oxygen involvement. This is confirmed by EPR experiments that carbon-centerd radicals are formed by light irradiation of tamoxifen and there is no singlet oxygen formation. Although superoxide radical is formed, it is not involved in DNA damage.
Figures

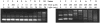

References
-
- Harwood KV. Advances in endocrine therapy for breast cancer: considering efficacy, safety, and quality of life. Clin J Oncology. 2004;8:629–637. - PubMed
-
- Shibutani S, Ravindernath A, Suzuki M, Terashima I, Sugarman SM, Grollman AP, Paearl ML. Identification of tamoxifen-DNA adducts in the endometrium of women treated with tamoxifen. Carcinogenesis. 2000;21:1461–1467. - PubMed
-
- Shibutani S, Reardon JT, Suzuki M, Sancar A. Excision of tamoxifen-DNA adducts by the human nucleotide excision repair system. Cancer Res. 2000;60:2607–2610. - PubMed
-
- Glatt H, Davis W, Meinl W, Hermersdörfer H, Venitt S, Phillips DH. Rat, but not human, sulfotransferase activates a tamoxifen metabolite to prodice DNA adducts and gene mutations in bacteria and mammalian cells in culture. Carcinogenesis. 1998;19:1709–1713. - PubMed
-
- Divi RL, Osborne MR, Hewer A, Phillips DH, Poirier MC. Tamoxifen-DNA adduct formation in rat liver determined by immunoassay and 32P-Postlabeling. Cancer Res. 1999;59:4829–4833. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
