The Diels-Alder-reaction with inverse-electron-demand, a very efficient versatile click-reaction concept for proper ligation of variable molecular partners
- PMID: 20046231
- PMCID: PMC2792734
- DOI: 10.7150/ijms.7.19
The Diels-Alder-reaction with inverse-electron-demand, a very efficient versatile click-reaction concept for proper ligation of variable molecular partners
Abstract
The ligation of active pharmaceutical ingredients (API) for working with image processing systems in diagnostics (MRT) attracts increasing notice and scientific interest. The Diels-Alder ligation Reaction with inverse electron demand (DAR(inv)) turns out to be an appropriate candidate. The DAR(inv) is characterized by a specific distribution of electrons of the diene and the corresponding dienophile counterpart. Whereas the reactants in the classical Diels-Alder Reaction feature electron-rich diene and electron-poor dienophile compounds, the DAR(inv) exhibits exactly the opposite distribution of electrons. Substituents with pushing electrones increase and, with pulling electrons reduce the electron density of the dienes as used in the DAR(inv).We report here that the DAR(inv) is an efficient route for coupling of multifunctional molecules like active peptides, re-formulated drugs or small molecules like the alkyalting agent temozolomide (TMZ). This is an example of our contribution to the "Click chemistry" technology. In this case TMZ is ligated by DAR(inv) as a cargo to transporter molecules facilitating the passage across the cell membranes into cells and subsequently into subcellular components like the cell nucleus by using address molecules. With such constructs we achieved high local concentrations at the desired target site of pharmacological action. The DAR(inv) ligation was carried out using the combination of several technologies, namely: the organic chemistry and the solid phase peptide synthesis which can produce 'tailored' solutions for questions not solely restricted to the medical diagnostics or therapy, but also result in functionalizations of various surfaces qualified amongst others also for array development.We like to acquaint you with the DAR(inv) and we like to exemplify that all ligation products were generated after a rapid and complete reaction in organic solutions at room temperature, in high purity, but also, hurdles and difficulties on the way to the TMZ-BioShuttle conjugate should be mentioned.With this report we would like to stimulate scientists working with the focus on "Click chemistry" to intensify research with this expanding DAR(inv )able to open the door for new solutions inconceivable so far.
Keywords: Adaptor Systems; Click-Chemistry; Cycloaddition; Diagnostics; Ligation chemistry; Linker Systems; Tetrazines; Therapy; Triazines; inverse Diels Alder Reaction.
Conflict of interest statement
Conflict of Interest: The authors have declared that no conflict of interest exists.
Figures
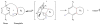
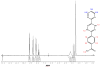

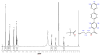


References
-
- Kohn M, Breinbauer R. The Staudinger ligation-a gift to chemical biology. Angew Chem Int Ed Engl. 2004;43:3106–16. - PubMed
-
- Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chem Int Ed Engl. 2001;40:2004–21. - PubMed
-
- Huisgen R. Theory of 1,3-Dipolar Cycloadditions. In: Padwa A, editor. 1,3-Dipolar Cycloaddition Chemistry. New York: Wiley; 1984. pp. 1–176.
-
- Rostovtsev VV, Green LG, Fokin VV. et al.A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective "ligation" of azides and terminal alkynes. Angew Chem Int Ed Engl. 2002;41:2596–9. - PubMed
-
- Chan TR, Hilgraf R, Sharpless KB. et al.Polytriazoles as copper(I)-stabilizing ligands in catalysis. Organic Letters. 2004;6:2853–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

