Withdrawal of immunosuppression in pediatric liver transplant recipients in Korea
- PMID: 20046418
- PMCID: PMC2796404
- DOI: 10.3349/ymj.2009.50.6.784
Withdrawal of immunosuppression in pediatric liver transplant recipients in Korea
Abstract
Purpose: We identified pediatric liver transplant recipients with successful withdrawal of immunosuppression who developed tolerance in Korea.
Materials and methods: Among 105 pediatric patients who received liver transplantation and were treated with tacrolimus-based immunosuppressive regimens, we selected five (4.8%) patients who had very low tacrolimus trough levels. Four of them were noncompliant with their medication and one was weaned off of immunosuppression due to life threatening posttransplant lymphoproliferative disorder. We reviewed the medical records with regard to the relationship of the donor-recipients, patient characteristics and prognosis, including liver histology, and compared our data with previous reports.
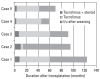
Results: Four patients received the liver transplantation from a parent donor and one patient from a cadaver donor. A trial of withdrawal of the immunosuppressant was started a median of 45 months after transplantation (range, 14 months to 60 months), and the period of follow up after weaning from the immunosuppressant was a median of 32 months (range, 14 months to 82 months). None of the five patients had rejection episodes after withdrawal of the immunosuppression; they maintained normal graft function for longer than 3 years (median, 38 months; range, 4 to 53 months). The histological findings of two grafts 64 and 32 months after weaning-off of the medication showed no evidence of chronic rejection.
Conclusion: The favorable markers for successful withdrawal of immunosuppression were 1) long-term (> 3 years) stable graft function, 2) no rejection for longer than 1 year after withdrawal of immunosuppression, 3) non-immune mediated liver diseases, and 4) pediatric patients.
Keywords: Pediatric liver transplantation; tacrolimus; withdrawal of immunosuppression.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures

Similar articles
-
De novo malignancies after liver transplantation: The effect of immunosuppression-personal data and review of literature.World J Gastroenterol. 2019 Sep 21;25(35):5356-5375. doi: 10.3748/wjg.v25.i35.5356. World J Gastroenterol. 2019. PMID: 31558879 Free PMC article.
-
Weaning of immunosuppression in living donor liver transplant recipients.Transplantation. 2001 Aug 15;72(3):449-54. doi: 10.1097/00007890-200108150-00016. Transplantation. 2001. PMID: 11502975
-
Weaning of immunosuppression in liver transplant recipients.Transplantation. 1997 Jan 27;63(2):243-9. doi: 10.1097/00007890-199701270-00012. Transplantation. 1997. PMID: 9020325 Free PMC article.
-
Minimization or withdrawal of immunosuppressants in pediatric liver transplant recipients.J Pediatr Surg. 2015 Dec;50(12):2128-33. doi: 10.1016/j.jpedsurg.2015.08.043. Epub 2015 Aug 28. J Pediatr Surg. 2015. PMID: 26377868
-
Conversion from cyclosporin to tacrolimus in paediatric liver transplant recipients.Paediatr Drugs. 2001;3(9):661-72. doi: 10.2165/00128072-200103090-00004. Paediatr Drugs. 2001. PMID: 11688597 Review.
Cited by
-
Translational lessons from a case of combined heart and liver transplantation for familial hypercholesterolemia 20 years post-operatively.J Cardiovasc Transl Res. 2012 Jun;5(3):351-8. doi: 10.1007/s12265-011-9311-1. Epub 2011 Sep 1. J Cardiovasc Transl Res. 2012. PMID: 21882079
-
Immunological Tolerance in Liver Transplant Recipients: Putative Involvement of Neuroendocrine-Immune Interactions.Cells. 2022 Jul 29;11(15):2327. doi: 10.3390/cells11152327. Cells. 2022. PMID: 35954171 Free PMC article. Review.
-
Efficacy of immunosuppression monotherapy after liver transplantation: a meta-analysis.World J Gastroenterol. 2014 Sep 14;20(34):12330-40. doi: 10.3748/wjg.v20.i34.12330. World J Gastroenterol. 2014. PMID: 25232269 Free PMC article. Review.
-
De novo malignancies after liver transplantation: The effect of immunosuppression-personal data and review of literature.World J Gastroenterol. 2019 Sep 21;25(35):5356-5375. doi: 10.3748/wjg.v25.i35.5356. World J Gastroenterol. 2019. PMID: 31558879 Free PMC article.
-
Strategies for Deliberate Induction of Immune Tolerance in Liver Transplantation: From Preclinical Models to Clinical Application.Front Immunol. 2020 Jul 31;11:1615. doi: 10.3389/fimmu.2020.01615. eCollection 2020. Front Immunol. 2020. PMID: 32849546 Free PMC article. Review.
References
-
- Lerut J, Sanchez-Fueyo A. An appraisal of tolerance in liver transplantation. Am J Transplant. 2006;6:1774–1780. - PubMed
-
- Devlin J, Doherty D, Thomson L, Wong T, Donaldson P, Portmann B, et al. Defining the outcome of immunosuppression withdrawal after liver transplantation. Hepatology. 1998;27:926–933. - PubMed
-
- Takatsuki M, Uemoto S, Inomata Y, Egawa H, Kiuchi T, Fujita S, et al. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation. 2001;72:449–454. - PubMed
-
- Thomson AW, Mazariegos GV, Reyes J, Donnenberg VS, Donnenberg AD, Bentlejewski C, et al. Monitoring the patient off immunosuppression. Conceptual framework for a proposed tolerance assay study in liver transplant recipients. Transplantation. 2001;72(8 Suppl):S13–S22. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

