Two doses of humanized anti-CD25 antibody in renal transplantation: a preliminary comparative study
- PMID: 20046574
- PMCID: PMC2715186
- DOI: 10.4161/mabs.1.1.7399
Two doses of humanized anti-CD25 antibody in renal transplantation: a preliminary comparative study
Abstract
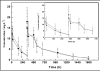
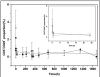
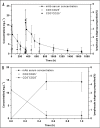
HuCD25mAb is a humanized anti-CD25 antibody which has the same amino acid sequence as daclizumab (Zenapax, Roche). HuCD25mAb is expressed in Chinese hamster ovary (CHO) cells while daclizumab is expressed in the NSO myeloma cell line. A comparative study was performed to evaluate the pharmacokinetics and pharmacodynamics between huCD25mAb and daclizumab in a two-dose regimen incorporating triple immunosuppressant treatment regimens (MMF, CsA and steroids). Fifteen patients were enrolled and randomized to receive intravenous infusion of either huCD25mAb (n = 10) or daclizumab (n = 5) at a dosage of 1 mg.kg(-1) on operation day 0 and post-operation day 14. Serum concentrations of huCD25mAb and daclizumab were measured by a validated competitive ELISA. Subgroups of CD3(+), CD25(+), CD4(+) and CD8(+) lymphocytes were monitored periodically by flow cytometry. The concentration-time curves of huCD25mAb and daclizumab were found to fit well to a one-compartment model. A significant decline of proportion (%) of CD3-CD25(+) and CD3(+)CD25(+) lymphocytes was observed 30 min after first infusion on day 0 (3.40 +/- 1.83 to 0.03 +/- 0.07, 3.35 +/- 2.02 to 0.37 +/- 0.49), and these levels remained low for at least 70 days (0.03 +/- 0.05, 0.31 +/- 0.47). All pharmacokinetic parameters of huCD25mAb seemed similar to those of daclizumab. The two-dose huCD25mAb regimen was as effective as daclizumab in rapidly achieving high therapeutic concentration in the treated patients, and a significant decrease of CD3(-)CD25(+) and CD3(+)CD25(+) lymphocytes was demonstrated. This suggests that two-dose regimen is feasible in maintaining host immunosuppression and may provide an effective and economical strategy for reducing incidence of acute graft rejection.
Keywords: CD25; enzyme immunoassay; flow cytometry; kidney transplantation; monoclonal antibody; pharmacokinetics.
Figures

Similar articles
-
Pharmacokinetics, pharmacodynamics, and immunodynamics of daclizumab in a two-dose regimen in liver transplantation.Transplantation. 2002 May 27;73(10):1640-6. doi: 10.1097/00007890-200205270-00020. Transplantation. 2002. PMID: 12042653 Clinical Trial.
-
Following anti-CD25 treatment, a functional CD4+CD25+ regulatory T-cell pool is present in renal transplant recipients.Am J Transplant. 2007 Jan;7(1):249-55. doi: 10.1111/j.1600-6143.2006.01604.x. Epub 2006 Nov 15. Am J Transplant. 2007. PMID: 17109733 Clinical Trial.
-
A multicenter, open-label, comparative trial of two daclizumab dosing strategies vs. no antibody induction in combination with tacrolimus, mycophenolate mofetil, and steroids for the prevention of acute rejection in simultaneous kidney-pancreas transplant recipients: interim analysis.Clin Transplant. 2002 Feb;16(1):60-8. doi: 10.1034/j.1399-0012.2002.00108.x. Clin Transplant. 2002. PMID: 11982617 Clinical Trial.
-
Daclizumab: a review of its use in the prevention of acute rejection in renal transplant recipients.Drugs. 1999 Dec;58(6):1029-42. doi: 10.2165/00003495-199958060-00006. Drugs. 1999. PMID: 10651389 Review.
-
Daclizumab: a review of its use in the management of organ transplantation.BioDrugs. 2001;15(11):745-73. doi: 10.2165/00063030-200115110-00005. BioDrugs. 2001. PMID: 11707149 Review.
Cited by
-
Tolerability, pharmacokinetics and pharmacodynamics of CMAB007, a humanized anti-immunoglobulin E monoclonal antibody, in healthy Chinese subjects.MAbs. 2012 Jan-Feb;4(1):110-9. doi: 10.4161/mabs.4.1.18349. MAbs. 2012. PMID: 22327434 Free PMC article. Clinical Trial.
-
Humanized anti-CD25 monoclonal antibody treatment of steroid-refractory acute graft-versus-host disease: a Chinese single-center experience in a group of 64 patients.Blood Cancer J. 2015 Apr 17;5(4):e308. doi: 10.1038/bcj.2015.33. Blood Cancer J. 2015. PMID: 25885428 Free PMC article. No abstract available.
-
Prediction of Clearance of Monoclonal and Polyclonal Antibodies and Non-Antibody Proteins in Children: Application of Allometric Scaling.Antibodies (Basel). 2020 Aug 5;9(3):40. doi: 10.3390/antib9030040. Antibodies (Basel). 2020. PMID: 32764408 Free PMC article.
References
-
- Vincenti F, Kirkman R, Light S, Bumgardner G, Pescovitz M, Halloran P, et al. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. N Engl J Med. 1998;338:161–165. - PubMed
-
- Nashan B, Light S, Hardie IR, Lin A, Johnson JR. Reduction of acute renal allograft rejection by daclizumab. Transplantation. 1999;67:110–115. - PubMed
-
- Vincenti F, Nashan B, Light S. Daclizumab: outcome of phase III trials and mechanism of action. Transplant Proc. 1998;30:2155–2158. - PubMed
-
- Pescovitz MD, Bumgardner G, Gaston RS, Kirkman RL, Light S, Patel IH, et al. Pharmacokinetics of daclizumab and mycophenolate mofetil with cyclosporine and steroids in renal transplantation. Clin Transplant. 2003;17:511–517. - PubMed
-
- Ciancio G, Mattiazzi A, Roth D, Kupin W, Miller J, Burker GW. The use of daclizumab as induction therapy in combination with tacrolimus and mycophenolate mofetil in recipients with previous transplants. Clin Transplant. 2003;17:428–432. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
