Clinical evaluation of a transcutaneous interrogated fluorescence lifetime-based microsensor for continuous glucose reading
- PMID: 20046654
- PMCID: PMC2769858
- DOI: 10.1177/193229680900300111
Clinical evaluation of a transcutaneous interrogated fluorescence lifetime-based microsensor for continuous glucose reading
Abstract
Background: Continuous glucose monitoring is presently used worldwide. Accuracy, precision, durability, invasiveness, and lack of drift of sensors and lag time are key parameters essential to these systems. This article describes a new online minimally invasive biodegradable microsensor for optical, transcutaneous interrogation, which has at least 14 days of functionality.
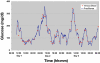
Method: Studies were performed in vitro and in vivo on pigs, as well as on type 1 diabetic humans. Functionality has been ensured in laboratory settings, and precision and durability have been tested in vivo. During in vivo studies, venous blood samples were used as reference. Results were based on one single point calibration per experiment.
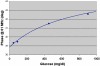
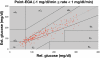
Results: Excellent stability was found in 14-day in vitro trials as well as in vivo in up to 70-hour trials. The overall median relative absolute difference of type 1 diabetic patients was 11.4%. Error grid analysis showed 97.7% of all values in the A+B zone. Comparable results were found in animal studies. No sensor drift was observed in any trial.
Conclusion: Results point toward the possibility of developing a stable and precise minimally invasive glucose reader for at least 2 weeks of continuous use.
Keywords: accuracy; continuous glucose monitoring; fluorescence; lifetime; variability.
© Diabetes Technology Society
Figures

Similar articles
-
Continuous glucose monitoring in subcutaneous tissue using factory-calibrated sensors: a pilot study.Diabetes Technol Ther. 2010 Aug;12(8):591-7. doi: 10.1089/dia.2010.0051. Diabetes Technol Ther. 2010. PMID: 20615099
-
Amperometric glucose sensors: sources of error and potential benefit of redundancy.J Diabetes Sci Technol. 2010 Jan 1;4(1):221-5. doi: 10.1177/193229681000400127. J Diabetes Sci Technol. 2010. PMID: 20167187 Free PMC article. Review.
-
Influence of time point of calibration on accuracy of continuous glucose monitoring in individuals with type 1 diabetes.Diabetes Technol Ther. 2012 Jul;14(7):583-8. doi: 10.1089/dia.2011.0271. Epub 2012 Apr 18. Diabetes Technol Ther. 2012. PMID: 22512266
-
New features and performance of a next-generation SEVEN-day continuous glucose monitoring system with short lag time.Diabetes Technol Ther. 2009 Dec;11(12):749-55. doi: 10.1089/dia.2009.0075. Diabetes Technol Ther. 2009. PMID: 20001675 Clinical Trial.
-
Biosensors for real-time in vivo measurements.Biosens Bioelectron. 2005 Jun 15;20(12):2388-403. doi: 10.1016/j.bios.2004.12.003. Epub 2005 Jan 15. Biosens Bioelectron. 2005. PMID: 15854814 Review.
Cited by
-
Options for the Development of Noninvasive Glucose Monitoring: Is Nanotechnology an Option to Break the Boundaries?J Diabetes Sci Technol. 2016 May 3;10(3):782-9. doi: 10.1177/1932296815616133. Print 2016 May. J Diabetes Sci Technol. 2016. PMID: 26581879 Free PMC article. Review.
-
Bibliometric analysis of research on the utilization of nanotechnology in diabetes mellitus and its complications.Nanomedicine (Lond). 2024 Jul 2;19(16):1449-1469. doi: 10.1080/17435889.2024.2358741. Epub 2024 Aug 9. Nanomedicine (Lond). 2024. PMID: 39121376 Free PMC article.
-
Products for Monitoring Glucose Levels in the Human Body With Noninvasive Optical, Noninvasive Fluid Sampling, or Minimally Invasive Technologies.J Diabetes Sci Technol. 2022 Jan;16(1):168-214. doi: 10.1177/19322968211007212. Epub 2021 Jun 13. J Diabetes Sci Technol. 2022. PMID: 34120487 Free PMC article. Review.
-
Clinical Performance Evaluation of Continuous Glucose Monitoring Systems: A Scoping Review and Recommendations for Reporting.J Diabetes Sci Technol. 2023 Nov;17(6):1506-1526. doi: 10.1177/19322968231190941. Epub 2023 Aug 20. J Diabetes Sci Technol. 2023. PMID: 37599389 Free PMC article.
-
High Affinity Mannotetraose as an Alternative to Dextran in ConA Based Fluorescent Affinity Glucose Assay Due to Improved FRET Efficiency.ACS Sens. 2016 May 27;1(5):584-590. doi: 10.1021/acssensors.5b00304. Epub 2016 Mar 16. ACS Sens. 2016. PMID: 28529973 Free PMC article.
References
-
- Robinson MR, Eaton RP, Haaland DM, Koepp GW, Thomas EV, Stallard BR, Robinson PL. Noninvasive glucose monitoring in diabetic patients: a preliminary evaluation. Clin Chem. 1992;38(9):1618–1622. - PubMed
-
- Small GW, Arnold MA, Marquardt LA. Strategies for coupling digital filtering with partial least-squares regression: application to the determination of glucose in plasma by Fourier transform near-infrared spectroscopy. Anal Chem. 1993;65(22):3279–3289. - PubMed
-
- Pfutzner A, Caduff A, Larbig M, Schrepfer T, Forst T. Impact of posture and fixation technique on impedance spectroscopy used for continuous and noninvasive glucose monitoring. Diabetes Technol Ther. 2004;6(4):435–441. - PubMed
-
- Potts RO, Tamada JA, Tierney MJ. Glucose monitoring by reverse iontophoresis. Diabetes Metab Res Rev. 2002;18(Suppl 1):S49–S53. - PubMed
-
- Ehwald R, Ballerstadt R, Dautzenberg H. Viscosimetric affinity assay. Anal Biochem. 1996;234(1):1–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

