Incorporating a generic model of subcutaneous insulin absorption into the AIDA v4 diabetes simulator. 3. Early plasma insulin determinations
- PMID: 20046665
- PMCID: PMC2769853
- DOI: 10.1177/193229680900300123
Incorporating a generic model of subcutaneous insulin absorption into the AIDA v4 diabetes simulator. 3. Early plasma insulin determinations
Abstract
Introduction: AIDA is an interactive educational diabetes simulator that has been available without charge via the Internet for over 12 years. Recent articles have described the incorporation of a novel generic model of insulin absorption into AIDA as a way of enhancing its capabilities. The basic model components to be integrated have been overviewed, with the aim being to provide simulations of regimens utilizing insulin analogues, as well as insulin doses greater than 40 IU (the current upper limit within the latest release of AIDA [v4.3a]). Some preliminary calculated insulin absorption results have also recently been described.
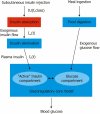
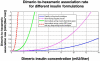
Methods: This article presents the first simulated plasma insulin profiles from the integration of the generic subcutaneous insulin absorption model, and the currently implemented model in AIDA for insulin disposition. Insulin absorption has been described by the physiologically based model of Tarín and colleagues. A single compartment modeling approach has been used to specify how absorbed insulin is distributed in, and eliminated from, the human body. To enable a numerical solution of the absorption model, a spherical subcutaneous depot for the injected insulin dose has been assumed and spatially discretized into shell compartments with homogeneous concentrations, having as its center the injection site. The number of these compartments will depend on the dose and type of insulin. Insulin inflow arises as the sum of contributions to the different shells. For this report the first bench testing of plasma insulin determinations has been done.
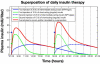
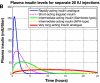
Results: Simulated plasma insulin profiles are provided for currently available insulin preparations, including a rapidly acting insulin analogue (e.g., lispro/Humalog or aspart/Novolog), a short-acting (regular) insulin preparation (e.g., Actrapid), intermediate-acting insulins (both Semilente and neutral protamine Hagedorn types), and a very long-acting insulin analogue (e.g., glargine/Lantus), as well as for insulin doses up to 50 IU.
Discussion: The methodology to be adopted for implementing the generic absorption model within AIDA has been overviewed, and the first plasma insulin profiles based on this approach have been demonstrated. Ideas for future work and development are discussed. It is expected that an updated release of AIDA (v4.5), based on this collaborative approach, will become available for free--in due course--via the www.2aida.org Web site. Readers who wish to be informed when the new software is launched can join the very low volume AIDA announcement list by sending a blank email note to subscribe@2aida.org.
Keywords: absorption; computer; diabetes; insulin; model; simulation; software.
© Diabetes Technology Society
Figures




Similar articles
-
Incorporating a generic model of subcutaneous insulin absorption into the AIDA v4 diabetes simulator: 2. preliminary bench testing.J Diabetes Sci Technol. 2007 Sep;1(5):780-93. doi: 10.1177/193229680700100525. J Diabetes Sci Technol. 2007. PMID: 19885148 Free PMC article.
-
Incorporating a generic model of subcutaneous insulin absorption into the AIDA v4 diabetes simulator: 1. a prospective collaborative development plan.J Diabetes Sci Technol. 2007 May;1(3):423-35. doi: 10.1177/193229680700100317. J Diabetes Sci Technol. 2007. PMID: 19885100 Free PMC article.
-
The freeware AIDA interactive educational diabetes simulator--http://www.2aida.org--(1). A download survey for AIDA v4.0.Med Sci Monit. 2001 May-Jun;7(3):504-15. Med Sci Monit. 2001. PMID: 11386033
-
Why people download the freeware AIDA v4.3a diabetes software program: a proof-of-concept semi-automated analysis.Diabetes Technol Ther. 2003;5(3):477-90. doi: 10.1089/152091503765692027. Diabetes Technol Ther. 2003. PMID: 12828835 Review.
-
Research use of the AIDA www.2aida.org diabetes software simulation program: a review--part 2. Generating simulated blood glucose data for prototype validation.Diabetes Technol Ther. 2003;5(4):641-51. doi: 10.1089/152091503322250668. Diabetes Technol Ther. 2003. PMID: 14511419 Review.
Cited by
-
Incorporating a generic model of subcutaneous insulin absorption into the AIDA v4 diabetes simulator: 2. preliminary bench testing.J Diabetes Sci Technol. 2007 Sep;1(5):780-93. doi: 10.1177/193229680700100525. J Diabetes Sci Technol. 2007. PMID: 19885148 Free PMC article.
-
Evaluation of pharmacokinetic model designs for subcutaneous infusion of insulin aspart.J Pharmacokinet Pharmacodyn. 2017 Oct;44(5):477-489. doi: 10.1007/s10928-017-9535-z. Epub 2017 Aug 22. J Pharmacokinet Pharmacodyn. 2017. PMID: 28831695 Clinical Trial.
-
Dynamic Interactive Educational Diabetes Simulations Using the World Wide Web: An Experience of More Than 15 Years with AIDA Online.Int J Endocrinol. 2014;2014:692893. doi: 10.1155/2014/692893. Epub 2014 Jan 6. Int J Endocrinol. 2014. PMID: 24511312 Free PMC article.
References
-
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. - PubMed
-
- Lehmann ED, Deutsch T. A physiological model of glucose-insulin interaction. IEEE EMBS Proceedings. 13th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 1991. pp. 2274–2275.
-
- Lehmann ED, Deutsch T. A physiological model of glucose-insulin interaction in type I diabetes mellitus. J Biomed Eng. 1992;14(3):235–242. - PubMed
-
- Lehmann ED. Diabetes moves onto the Internet. Lancet. 1996:347–1542.
-
- Lehmann ED. University of London/Imperial College of Science, Technology and Medicine; 2004. Development, evaluation, and usage of ‘AIDA’– an interactive educational diabetes simulator. Ph. D. Thesis.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

