Abnormal placental development and early embryonic lethality in EpCAM-null mice
- PMID: 20046825
- PMCID: PMC2796178
- DOI: 10.1371/journal.pone.0008543
Abnormal placental development and early embryonic lethality in EpCAM-null mice
Abstract
Background: EpCAM (CD326) is encoded by the tacstd1 gene and expressed by a variety of normal and malignant epithelial cells and some leukocytes. Results of previous in vitro experiments suggested that EpCAM is an intercellular adhesion molecule. EpCAM has been extensively studied as a potential tumor marker and immunotherapy target, and more recent studies suggest that EpCAM expression may be characteristic of cancer stem cells.
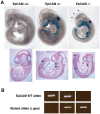
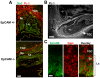
Methodology/principal findings: To gain insights into EpCAM function in vivo, we generated EpCAM -/- mice utilizing an embryonic stem cell line with a tacstd1 allele that had been disrupted. Gene trapping resulted in a protein comprised of the N-terminus of EpCAM encoded by 2 exons of the tacstd1 gene fused in frame to betageo. EpCAM +/- mice were viable and fertile and exhibited no obvious abnormalities. Examination of EpCAM +/- embryos revealed that betageo was expressed in several epithelial structures including developing ears (otocysts), eyes, branchial arches, gut, apical ectodermal ridges, lungs, pancreas, hair follicles and others. All EpCAM -/- mice died in utero by E12.5, and were small, developmentally delayed, and displayed prominent placental abnormalities. In developing placentas, EpCAM was expressed throughout the labyrinthine layer and by spongiotrophoblasts as well. Placentas of EpCAM -/- embryos were compact, with thin labyrinthine layers lacking prominent vascularity. Parietal trophoblast giant cells were also dramatically reduced in EpCAM -/- placentas.
Conclusion: EpCAM was required for differentiation or survival of parietal trophoblast giant cells, normal development of the placental labyrinth and establishment of a competent maternal-fetal circulation. The findings in EpCAM-reporter mice suggest involvement of this molecule in development of vital organs including the gut, kidneys, pancreas, lungs, eyes, and limbs.
Conflict of interest statement
Figures




References
-
- Edwards DP, Grzyb KT, Dressler LG, Mansel RE, Zava DT, et al. Monoclonal antibody identification and characterization of a Mr 43,000 membrane glycoprotein associated with human breast cancer. Cancer Res. 1986;46:1306–1317. - PubMed
-
- Ross AH, Herlyn D, Iliopoulos D, Koprowski H. Isolation and characterization of a carcinoma-associated antigen. Biochem Biophys Res Commun. 1986;135:297–303. - PubMed
-
- Bergsagel PL, Victor-Kobrin C, Timblin CR, Trepel J, Kuehl WM. A murine cDNA encodes a pan-epithelial glycoprotein that is also expressed on plasma cells. J Immunol. 1992;148:590–596. - PubMed
-
- Borkowski TA, Nelson AJ, Farr AG, Udey MC. Expression of gp40, the murine homologue of human epithelial cell adhesion molecule (Ep-CAM), by murine dendritic cells. Eur J Immunol. 1996;26:110–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

