Mechanisms of c-myc degradation by nickel compounds and hypoxia
- PMID: 20046830
- PMCID: PMC2797325
- DOI: 10.1371/journal.pone.0008531
Mechanisms of c-myc degradation by nickel compounds and hypoxia
Abstract
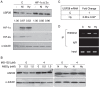
Nickel (Ni) compounds have been found to cause cancer in humans and animal models and to transform cells in culture. At least part of this effect is mediated by stabilization of hypoxia inducible factor (HIF1a) and activating its downstream signaling. Recent studies reported that hypoxia signaling might either antagonize or enhance c-myc activity depending on cell context. We investigated the effect of nickel on c-myc levels, and demonstrated that nickel, hypoxia, and other hypoxia mimetics degraded c-myc protein in a number of cancer cells (A549, MCF-7, MDA-453, and BT-474). The degradation of the c-Myc protein was mediated by the 26S proteosome. Interestingly, knockdown of both HIF-1alpha and HIF-2alpha attenuated c-Myc degradation induced by Nickel and hypoxia, suggesting the functional HIF-1alpha and HIF-2alpha was required for c-myc degradation. Further studies revealed two potential pathways mediated nickel and hypoxia induced c-myc degradation. Phosphorylation of c-myc at T58 was significantly increased in cells exposed to nickel or hypoxia, leading to increased ubiquitination through Fbw7 ubiquitin ligase. In addition, nickel and hypoxia exposure decreased USP28, a c-myc de-ubiquitinating enzyme, contributing to a higher steady state level of c-myc ubiquitination and promoting c-myc degradation. Furthermore, the reduction of USP28 protein by hypoxia signaling is due to both protein degradation and transcriptional repression. Nickel and hypoxia exposure significantly increased the levels of dimethylated H3 lysine 9 at the USP28 promoter and repressed its expression. Our study demonstrated that Nickel and hypoxia exposure increased c-myc T58 phosphorylation and decreased USP28 protein levels in cancer cells, which both lead to enhanced c-myc ubiquitination and proteasomal degradation.
Conflict of interest statement
Figures





References
-
- Miller AC, Mog S, McKinney L, Luo L, Allen J, et al. Neoplastic transformation of human osteoblast cells to the tumorigenic phenotype by heavy metal-tungsten alloy particles: induction of genotoxic effects. Carcinogenesis. 2001;22:115–125. - PubMed
-
- Kuper CF, Woutersen RA, Slootweg PJ, Feron VJ. Carcinogenic response of the nasal cavity to inhaled chemical mixtures. Mutat Res. 1997;380:19–26. - PubMed
-
- Kerckaert GA, LeBoeuf RA, Isfort RJ. Use of the Syrian hamster embryo cell transformation assay for determining the carcinogenic potential of heavy metal compounds. Fundam Appl Toxicol. 1996;34:67–72. - PubMed
-
- Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

