Bile acids in treatment of ocular disease
- PMID: 20046852
- PMCID: PMC2798994
- DOI: 10.1007/s12177-009-9030-x
Bile acids in treatment of ocular disease
Abstract
Bear bile has been included in Asian pharmacopeias for thousands of years in treatment of several diseases, ranging from sore throat to hemorrhoids. The hydrophilic bile acids tauroursodeoxycholic acid (TUDCA) and ursodeoxycholic acid (UDCA) are the major bile acids of bear bile. Both of these are available as synthetic formulations and are approved by the health administrations of several countries for treatment of cirrhosis and gallstones. This review briefly covers the use of bear bile in Traditional Chinese Medicine, bile acid physiology, approved use of UDCA and TUDCA in Western medicine, and recent research exploring their neuroprotective properties, including in models of ocular disease.
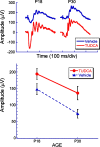
Figures








References
-
- Cidian Z. Dictionary of traditional Chinese medicine. Shanghai: Shanghai Science and Technology Press; 2004.
-
- Lee Y-J. The use of bear bile as medicine versus tonic. In: Williamson DF and Phipps MJ, editors. Proceedings of the Third International Symposium on the Trade in Bear Parts. Seoul: TRAFFIC East Asia; 1999. p. 122–126
-
- Gabriel GG.A bitter medicine: the use of bear bile in China. In: Williamson DF and Phipps MJ, editors. Proceedings of the Third International Symposium on the Trade in Bear Parts. Seoul: TRAFFIC East Asia; 1999. p. 116–120.
-
- Hanson DG. Poaching: Oriental demand for undgam and paws decimates California bears. Audubon. 1983;85:127–128.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
