Cellular levels of HIV unspliced RNA from patients on combination antiretroviral therapy with undetectable plasma viremia predict the therapy outcome
- PMID: 20046870
- PMCID: PMC2795168
- DOI: 10.1371/journal.pone.0008490
Cellular levels of HIV unspliced RNA from patients on combination antiretroviral therapy with undetectable plasma viremia predict the therapy outcome
Abstract
Background: Combination antiretroviral therapy (cART), the standard of care for HIV-1 infection, is considered to be successful when plasma viremia remains below the detection limit of commercial assays. Yet, cART fails in a substantial proportion of patients after the apparent success. No laboratory markers are known that are predictive of cART outcome in initial responders during the period of undetectable plasma viremia.
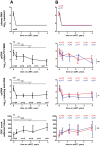
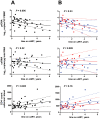
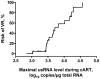
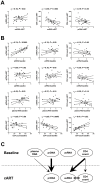
Methodology/principal findings: Here, we report the results of a retrospective longitudinal study of twenty-six HIV-infected individuals who initially responded to cART by having plasma viremia suppressed to <50 copies/ml. Eleven of these patients remained virologically suppressed, whereas fifteen experienced subsequent cART failure. Using sensitive methods based on seminested real-time PCR, we measured the levels of HIV-1 proviral (pr) DNA, unspliced (us) RNA, and multiply spliced RNA in the peripheral blood mononuclear cells (PBMC) of these patients at multiple time points during the period of undetectable plasma viremia on cART. Median under-therapy level of usRNA was significantly higher (0.43 log(10) difference, P = 0.0015) in patients who experienced subsequent cART failure than in successfully treated patients. In multivariate analysis, adjusted for baseline CD4(+) counts, prior ART experience, and particular cART regimens, the maximal usRNA level under therapy was the best independent predictor of subsequent therapy failure (adjusted odds ratio [95% CI], 24.4 [1.5-389.5], P = 0.024). The only other factor significantly associated with cART failure was prior ART experience (adjusted odds ratio [95% CI], 12.3 [1.1-138.4], P = 0.042). Levels of usRNA under cART inversely correlated with baseline CD4(+) counts (P = 0.0003), but did not correlate with either baseline usRNA levels or levels of prDNA under therapy.
Conclusion: Our data demonstrate that the level of HIV-1 usRNA in PBMC, measured in cART-treated patients with undetectable plasma viremia, is a strong predictive marker for the outcome of therapy.
Conflict of interest statement
Figures
References
-
- Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. - PubMed
-
- Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. - PubMed
-
- Weverling GJ, Lange JM, Jurriaans S, Prins JM, Lukashov VV, et al. Alternative multidrug regimen provides improved suppression of HIV-1 replication over triple therapy. AIDS. 1998;12:F117–F122. - PubMed
-
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. - PubMed
-
- Walensky RP, Paltiel AD, Losina E, Mercincavage LM, Schackman BR, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194:11–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

