Histidine-rich glycoprotein can prevent development of mouse experimental glioblastoma
- PMID: 20046875
- PMCID: PMC2795204
- DOI: 10.1371/journal.pone.0008536
Histidine-rich glycoprotein can prevent development of mouse experimental glioblastoma
Abstract
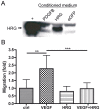
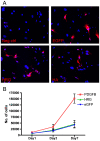
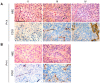
Extensive angiogenesis, formation of new capillaries from pre-existing blood vessels, is an important feature of malignant glioma. Several antiangiogenic drugs targeting vascular endothelial growth factor (VEGF) or its receptors are currently in clinical trials as therapy for high-grade glioma and bevacizumab was recently approved by the FDA for treatment of recurrent glioblastoma. However, the modest efficacy of these drugs and emerging problems with anti-VEGF treatment resistance welcome the development of alternative antiangiogenic therapies. One potential candidate is histidine-rich glycoprotein (HRG), a plasma protein with antiangiogenic properties that can inhibit endothelial cell adhesion and migration. We have used the RCAS/TV-A mouse model for gliomas to investigate the effect of HRG on brain tumor development. Tumors were induced with platelet-derived growth factor-B (PDGF-B), in the presence or absence of HRG. We found that HRG had little effect on tumor incidence but could significantly inhibit the development of malignant glioma and completely prevent the occurrence of grade IV tumors (glioblastoma).
Conflict of interest statement
Figures

Similar articles
-
Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment.Am J Pathol. 2003 Apr;162(4):1083-93. doi: 10.1016/S0002-9440(10)63905-3. Am J Pathol. 2003. PMID: 12651601 Free PMC article.
-
Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor.Cancer Res. 2006 Aug 15;66(16):7843-8. doi: 10.1158/0008-5472.CAN-06-1010. Cancer Res. 2006. PMID: 16912155
-
A tricistronic retroviral vector expressing natural antiangiogenic factors inhibits angiogenesis in vitro, but is not able to block tumor progression in vivo.Gene Ther. 2002 Feb;9(4):297-302. doi: 10.1038/sj.gt.3301652. Gene Ther. 2002. PMID: 11896469
-
Vascular morphology and angiogenesis in glial tumors.Exp Toxicol Pathol. 1995 May;47(2-3):89-94. doi: 10.1016/S0940-2993(11)80292-7. Exp Toxicol Pathol. 1995. PMID: 7580112 Review.
-
Bevacizumab in high-grade gliomas: past, present, and future.Expert Rev Anticancer Ther. 2015 Apr;15(4):387-97. doi: 10.1586/14737140.2015.1028376. Expert Rev Anticancer Ther. 2015. PMID: 25797685 Review.
Cited by
-
Multifaceted Role of the Placental Growth Factor (PlGF) in the Antitumor Immune Response and Cancer Progression.Int J Mol Sci. 2019 Jun 18;20(12):2970. doi: 10.3390/ijms20122970. Int J Mol Sci. 2019. PMID: 31216652 Free PMC article. Review.
-
Expression of FXR and HRG and their clinicopathological significance in benign and malignant pancreatic lesions.Int J Clin Exp Pathol. 2019 Jun 1;12(6):2111-2120. eCollection 2019. Int J Clin Exp Pathol. 2019. PMID: 31934033 Free PMC article.
-
Complex oncogenic signaling networks regulate brain tumor-initiating cells and their progenies: pivotal roles of wild-type EGFR, EGFRvIII mutant and hedgehog cascades and novel multitargeted therapies.Brain Pathol. 2011 Sep;21(5):479-500. doi: 10.1111/j.1750-3639.2011.00505.x. Epub 2011 Jul 7. Brain Pathol. 2011. PMID: 21615592 Free PMC article. Review.
-
Hsp70 and Calcitonin Receptor Protein in Extracellular Vesicles from Glioblastoma Multiforme: Biomarkers with Putative Roles in Carcinogenesis and Potential for Differentiating Tumor Types.Int J Mol Sci. 2024 Mar 18;25(6):3415. doi: 10.3390/ijms25063415. Int J Mol Sci. 2024. PMID: 38542389 Free PMC article.
-
Comprehensive characterization of glioblastoma tumor tissues for biomarker identification using mass spectrometry-based label-free quantitative proteomics.Physiol Genomics. 2014 Jul 1;46(13):467-81. doi: 10.1152/physiolgenomics.00034.2014. Epub 2014 May 6. Physiol Genomics. 2014. PMID: 24803679 Free PMC article.
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. - PubMed
-
- Plate KH, Breier G, Weich HA, Mennel HD, Risau W. Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer. 1994;59:520–529. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

