Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction
- PMID: 20046878
- PMCID: PMC2795856
- DOI: 10.1371/journal.pone.0008443
Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction
Abstract
Background: Differentiation of human embryonic stem cells into endothelial cells (hESC-ECs) has the potential to provide an unlimited source of cells for novel transplantation therapies of ischemic diseases by supporting angiogenesis and vasculogenesis. However, the endothelial differentiation efficiency of the conventional embryoid body (EB) method is low while the 2-dimensional method of co-culturing with mouse embryonic fibroblasts (MEFs) require animal product, both of which can limit the future clinical application of hESC-ECs. Moreover, to fully understand the beneficial effects of stem cell therapy, investigators must be able to track the functional biology and physiology of transplanted cells in living subjects over time.
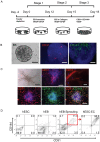
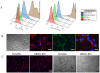
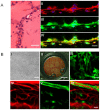
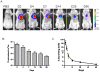
Methodology: In this study, we developed an extracellular matrix (ECM) culture system for increasing endothelial differentiation and free from contaminating animal cells. We investigated the transcriptional changes that occur during endothelial differentiation of hESCs using whole genome microarray, and compared to human umbilical vein endothelial cells (HUVECs). We also showed functional vascular formation by hESC-ECs in a mouse dorsal window model. Moreover, our study is the first so far to transplant hESC-ECs in a myocardial infarction model and monitor cell fate using molecular imaging methods.
Conclusion: Taken together, we report a more efficient method for derivation of hESC-ECs that express appropriate patterns of endothelial genes, form functional vessels in vivo, and improve cardiac function. These studies suggest that hESC-ECs may provide a novel therapy for ischemic heart disease in the future.
Conflict of interest statement
Figures



References
-
- Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ Res. 2005;96:151–163. - PubMed
-
- Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–856. - PubMed
-
- Huang PP, Yang XF, Li SZ, Wen JC, Zhang Y, et al. Randomised comparison of G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thromb Haemost. 2007;98:1335–1342. - PubMed
-
- Yang C, Zhang ZH, Li ZJ, Yang RC, Qian GQ, et al. Enhancement of neovascularization with cord blood CD133+ cell-derived endothelial progenitor cell transplantation. Thromb Haemost. 2004;91:1202–1212. - PubMed
-
- Heeschen C, Chang E, Aicher A, Cooke JP. Endothelial progenitor cells participate in nicotine-mediated angiogenesis. J Am Coll Cardiol. 2006;48:2553–2560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

