Absorption, Conjugation and Efflux of the Flavonoids, Kaempferol and Galangin, Using the Intestinal CACO-2/TC7 Cell Model
- PMID: 20046888
- PMCID: PMC2765672
- DOI: 10.1016/j.jff.2008.09.011
Absorption, Conjugation and Efflux of the Flavonoids, Kaempferol and Galangin, Using the Intestinal CACO-2/TC7 Cell Model
Abstract
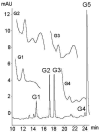
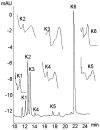
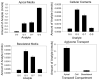
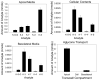
Flavonoids are biologically active compounds in food with potential health effects. We have used the Caco-2 cell monolayer model to study the absorption and metabolism of two flavonols, a class of flavonoids, specifically kaempferol and galangin. Metabolism experiments allowed identification of 5 kaempferol conjugates: 3-, 7- and 4'-glucuronide, a sulphate and a glucurono-sulphate; and 4 galangin conjugates: 3-, 5- and 7-glucuronides, and a sulphate, using specific enzyme hydrolysis, HPLC-MS, and HPLC with post column metal complexation/tandem MS. Transport studies showed that the flavonols were conjugated inside the cells then transported across the monolayer or effluxed back to the apical side. Sulphated conjugates were preferentially effluxed back to the apical side, whereas glucuronides were mostly transported to the basolateral side. For kaempferol, a small amount of the unconjugated aglycone permeated in both directions, indicating some passive diffusion. When kaempferol-3-glucuronide and quercetin7-sulphate were applied to either side of the cells, no permeation in either direction was observed, indicating that conjugates cannot re-cross the cell monolayer. Formation of apical kaempferol-7- and 4'-glucuronides was readily saturated, whereas formation of other conjugates at the apical side and all at the basolateral side increased with increasing concentration of kaempferol, implying different transporters are responsible at the apical and basolateral sides. The results highlight the important but complex metabolic changes occurring in flavonoids during absorption.
Figures
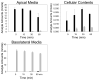
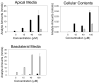
References
-
- Crespy V, Morand C, Besson C, Cotelle N, Vezin H, Demigne C, Remesy C. The splanchic metabolism of flavonoids highly differed according to the nature of the compound. American Journal of Physiology. 2003;284:980–988. - PubMed
-
- Crespy V, Morland C, Besson C, Manach C, Demigne C, Remesy C. Comparison of the intestinal absorption of quercetin, phloretin and their glucosides in rats. Journal of Nutrition. 2001;131:2109–2114. - PubMed
-
- Davis BD, Needs PW, Kroon PA, Brodbelt JS. Identification of isomeric flavonoid glucuronides in urine and plasma by metal complexation and LC-ESI-MS/MS. Journal of Mass Spectrometry. 2006;41:911–920. - PubMed
-
- Day AJ. Uptake an metabolism of dietary flavonoid glycosides. University of East Anglia (Institute of Food Research); Norwich. UK: 2000.
-
- Day AJ, Bao Y, Morgan MR, Williamson G. Conjugation position of quercetin glucuronides and effect on biological activity. Free Radicals in Biology and Medicine. 2000;29:1234–1243. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
