Excited-State Proton Transfer in Chiral Environments: Photoracemization of BINOLs
- PMID: 20046928
- PMCID: PMC2756819
- DOI: 10.1560/IJC.49.2.227
Excited-State Proton Transfer in Chiral Environments: Photoracemization of BINOLs
Abstract
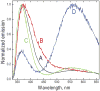
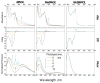
We have studied excited-state proton transfer (ESPT) from chiral proton donors to chiral and achiral acceptors. The key role of the exergonicity of the reaction and the transition-state position along the reaction coordinate for the existence of an enantiomeric effect was established. This effect was observed for "super" photoacids (ΔG ≪ 0) and vanished for endergonic reactions (ΔG > 0) where a "late" transition state similar to planar achiral binaphtholate anion occurs. As a result, photoracemization was observed, as confirmed by circular dichroism spectroscopy. The photoracemization effects were studied for several chiral photoacids (BINOLs and their ethers) and proton acceptors (amines, aminoalcohols, and water) using UV-vis, steady-state fluorescence, and time-resolved fluorescence spectroscopies. The nature of the solvent and the proton acceptor, as well as the chemical structure of the BINOL, played a pivotal role in the photochemical reactivity of the system. Two proposed pathways competed for photoracemization: excited-state inter- and intra-molecular proton transfer, the former being more effective. Irradiation of the dimethoxy BINOL derivative, which lacks an acidic proton and cannot undergo ESPT, produced no appreciable reaction or racemization.
Figures
Similar articles
-
Photoracemization-Based Viedma Ripening of a BINOL Derivative.Chemistry. 2020 Jan 16;26(4):839-844. doi: 10.1002/chem.201904382. Epub 2019 Dec 12. Chemistry. 2020. PMID: 31663650 Free PMC article.
-
Application of Super Photoacids in Controlling Dynamic Processes: Light-Triggering the Self-Propulsion of Oil Droplets.J Phys Chem B. 2022 Aug 25;126(33):6331-6337. doi: 10.1021/acs.jpcb.2c04020. Epub 2022 Aug 12. J Phys Chem B. 2022. PMID: 35959566
-
Unraveling the Detailed Mechanism of Excited-State Proton Transfer.Acc Chem Res. 2018 Jul 17;51(7):1681-1690. doi: 10.1021/acs.accounts.8b00172. Epub 2018 Jun 15. Acc Chem Res. 2018. PMID: 29906102
-
A dual experimental-theoretical perspective on ESPT photoacids and their challenges ahead.Chem Sci. 2024 Dec 2;16(4):1560-1596. doi: 10.1039/d4sc07148d. eCollection 2025 Jan 22. Chem Sci. 2024. PMID: 39759939 Free PMC article. Review.
-
Revealing the role of excited state proton transfer (ESPT) in excited state hydrogen transfer (ESHT): systematic study in phenol-(NH3) n clusters.Chem Sci. 2021 Feb 10;12(11):3836-3856. doi: 10.1039/d0sc06877b. Chem Sci. 2021. PMID: 34163653 Free PMC article. Review.
Cited by
-
Fluoride-driven 'turn on' ESPT in the binding with a novel benzimidazole-based sensor.Beilstein J Org Chem. 2015 Apr 24;11:563-7. doi: 10.3762/bjoc.11.61. eCollection 2015. Beilstein J Org Chem. 2015. PMID: 25977729 Free PMC article.
-
Circularly polarized luminescence by visible-light absorption in a chiral O-BODIPY dye: unprecedented design of CPL organic molecules from achiral chromophores.J Am Chem Soc. 2014 Mar 5;136(9):3346-9. doi: 10.1021/ja412294s. Epub 2014 Feb 21. J Am Chem Soc. 2014. PMID: 24524257 Free PMC article.
-
Regiodivergent Photocyclization of Dearomatized Acylphloroglucinols: Asymmetric Syntheses of (-)-Nemorosone and (-)-6-epi-Garcimultiflorone A.J Am Chem Soc. 2019 Jul 17;141(28):11315-11321. doi: 10.1021/jacs.9b05600. Epub 2019 Jul 2. J Am Chem Soc. 2019. PMID: 31264859 Free PMC article.
-
Photoracemization-Based Viedma Ripening of a BINOL Derivative.Chemistry. 2020 Jan 16;26(4):839-844. doi: 10.1002/chem.201904382. Epub 2019 Dec 12. Chemistry. 2020. PMID: 31663650 Free PMC article.
-
Ligand induced circular dichroism and circularly polarized luminescence in CdSe quantum dots.ACS Nano. 2013 Dec 23;7(12):11094-102. doi: 10.1021/nn404832f. Epub 2013 Nov 13. ACS Nano. 2013. PMID: 24200288 Free PMC article.
References
-
-
The preliminary results including the photoracemization scheme (Figure 1) were reported in parts at the following American Chemical Society National meetings: 225th, New Orleans (2003), PHYS 357; 229th, San Diego (2005), PHYS 456; 233th, Chicago (2007), PHYS 45; 234th, Boston (2007), PHYS 364.
-
-
- Solntsev KM, Tolbert LM, Cohen B, Huppert D, Hayashi Y, Feldman Yu. J Am Chem Soc. 2002;124:9046–9047. - PubMed
-
-
Recent reviews: Pu L. Chem Rev. 2004;104:1687–1716.Speranza M, Satta M, Piccirillo S, Rondino F, Paladini A, Giardini A, Filippi A, Catone D. Mass Spec Rev. 2005;24:588–610.Alkorta I, Picazo O, Elguero J. Curr Org Chem. 2006;10:695–714.Zehnacker A, Suhm MA. Angew Chem, Int Ed. 2008;47:6970–6992.
-
-
- Kyba EP, Gokel GW, de Jong F, Koga K, Sousa LR, Siegel MG, Kaplan L, Sogah GDY, Cram DJ. J Org Chem. 1977;42:4173–4184.
- Dobashi Y, Dobashi A, Iitaka Y. Tetrahedron Lett. 1994;35:9413–9416.
- Liu TJ, Chen YJ, Zhang KS, Wang D, Guo DW, Yang XZ. Chirality. 2001;13:595–600. - PubMed
- Ma L, White PS, Lin W. J Org Chem. 2002;67:7577–7586. - PubMed
- Xu Y, McCarroll M. J Photochem Photobiol A. 2006;178:50–56.
- Wang Q, Chen X, Tao L, Wang L, Xiao D, Yu XQ, Pu L. J Org Chem. 2007;72:97–101. - PMC - PubMed
- Upadhyay SP, Pissurlenkar RR, Coutinho EC, Karnik AV. J Org Chem. 2007;72:5709–5714. - PubMed
-
- Brunel JM. Chem Rev. 2005;105:857–898. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
