Excited-State Proton Transfer in Chiral Environments: Photoracemization of BINOLs
- PMID: 20046928
- PMCID: PMC2756819
- DOI: 10.1560/IJC.49.2.227
Excited-State Proton Transfer in Chiral Environments: Photoracemization of BINOLs
Abstract
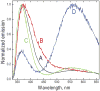
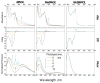
We have studied excited-state proton transfer (ESPT) from chiral proton donors to chiral and achiral acceptors. The key role of the exergonicity of the reaction and the transition-state position along the reaction coordinate for the existence of an enantiomeric effect was established. This effect was observed for "super" photoacids (ΔG ≪ 0) and vanished for endergonic reactions (ΔG > 0) where a "late" transition state similar to planar achiral binaphtholate anion occurs. As a result, photoracemization was observed, as confirmed by circular dichroism spectroscopy. The photoracemization effects were studied for several chiral photoacids (BINOLs and their ethers) and proton acceptors (amines, aminoalcohols, and water) using UV-vis, steady-state fluorescence, and time-resolved fluorescence spectroscopies. The nature of the solvent and the proton acceptor, as well as the chemical structure of the BINOL, played a pivotal role in the photochemical reactivity of the system. Two proposed pathways competed for photoracemization: excited-state inter- and intra-molecular proton transfer, the former being more effective. Irradiation of the dimethoxy BINOL derivative, which lacks an acidic proton and cannot undergo ESPT, produced no appreciable reaction or racemization.
Figures
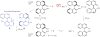
References
-
-
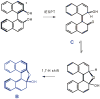
The preliminary results including the photoracemization scheme (Figure 1) were reported in parts at the following American Chemical Society National meetings: 225th, New Orleans (2003), PHYS 357; 229th, San Diego (2005), PHYS 456; 233th, Chicago (2007), PHYS 45; 234th, Boston (2007), PHYS 364.
-
-
- Solntsev KM, Tolbert LM, Cohen B, Huppert D, Hayashi Y, Feldman Yu. J Am Chem Soc. 2002;124:9046–9047. - PubMed
-
-
Recent reviews: Pu L. Chem Rev. 2004;104:1687–1716.Speranza M, Satta M, Piccirillo S, Rondino F, Paladini A, Giardini A, Filippi A, Catone D. Mass Spec Rev. 2005;24:588–610.Alkorta I, Picazo O, Elguero J. Curr Org Chem. 2006;10:695–714.Zehnacker A, Suhm MA. Angew Chem, Int Ed. 2008;47:6970–6992.
-
-
- Kyba EP, Gokel GW, de Jong F, Koga K, Sousa LR, Siegel MG, Kaplan L, Sogah GDY, Cram DJ. J Org Chem. 1977;42:4173–4184.
- Dobashi Y, Dobashi A, Iitaka Y. Tetrahedron Lett. 1994;35:9413–9416.
- Liu TJ, Chen YJ, Zhang KS, Wang D, Guo DW, Yang XZ. Chirality. 2001;13:595–600. - PubMed
- Ma L, White PS, Lin W. J Org Chem. 2002;67:7577–7586. - PubMed
- Xu Y, McCarroll M. J Photochem Photobiol A. 2006;178:50–56.
- Wang Q, Chen X, Tao L, Wang L, Xiao D, Yu XQ, Pu L. J Org Chem. 2007;72:97–101. - PMC - PubMed
- Upadhyay SP, Pissurlenkar RR, Coutinho EC, Karnik AV. J Org Chem. 2007;72:5709–5714. - PubMed
-
- Brunel JM. Chem Rev. 2005;105:857–898. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
