Origin of Substituent Effects in Edge-to-Face Aryl-Aryl Interactions
- PMID: 20046948
- PMCID: PMC2742475
- DOI: 10.1080/00268970802537614
Origin of Substituent Effects in Edge-to-Face Aryl-Aryl Interactions
Abstract
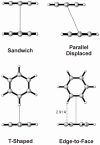
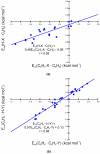
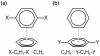
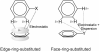
Substituent effects in the edge-to-face configuration of the benzene dimer have been studied using modern density functional theory. An accurate interaction potential energy curve has been computed for the unsubstituted dimer using ab initio methods with large basis sets. The recommended binding energy for the edge-to-face benzene dimer is 2.31 kcal mol(-1), estimated at the counterpoise-corrected CCSD(T)/aug-cc-pVTZ level of theory. For both edge-ring and face-ring-substituted dimers, interaction energies correlate with sigma(m) for the substituents, indicating that substituent effects can be understood qualitatively in terms of simple electrostatic effects, although in the latter case dispersion results in some scatter in the data. In contrast to prevailing models of substituent effects in benzene dimers, polarization of the pi-system of the substituted ring does not induce substituent effects. For edge-ring-substituted dimers, substituent effects arise from differential electrostatic interactions between the hydrogens on the substituted ring and the pi-cloud of the face ring and direct interactions of the substituents with the unsubstituted ring. For face-ring-substituted dimers, substituent effects arise from direct electrostatic and dispersion interactions of the substituents with the edge ring. Substituents with sigma(m) > 0.12 favor edge ring substitution while for sigma(m) < 0.12 substitution on the face ring is preferred.
Figures

Similar articles
-
Substituent effects in the benzene dimer are due to direct interactions of the substituents with the unsubstituted benzene.J Am Chem Soc. 2008 Aug 20;130(33):10854-5. doi: 10.1021/ja802849j. Epub 2008 Jul 25. J Am Chem Soc. 2008. PMID: 18652453 Free PMC article.
-
Substituent effects on the edge-to-face aromatic interactions.J Am Chem Soc. 2005 Mar 30;127(12):4530-7. doi: 10.1021/ja037454r. J Am Chem Soc. 2005. PMID: 15783237
-
Are anion/pi interactions actually a case of simple charge-dipole interactions?J Phys Chem A. 2010 Aug 26;114(33):8658-64. doi: 10.1021/jp1010549. J Phys Chem A. 2010. PMID: 20433187 Free PMC article.
-
Understanding substituent effects in noncovalent interactions involving aromatic rings.Acc Chem Res. 2013 Apr 16;46(4):1029-38. doi: 10.1021/ar300109n. Epub 2012 Jun 22. Acc Chem Res. 2013. PMID: 22725832
-
Ab initio study of substituent effects in the interactions of dimethyl ether with aromatic rings.Phys Chem Chem Phys. 2008 May 21;10(19):2695-705. doi: 10.1039/b718722j. Epub 2008 Mar 17. Phys Chem Chem Phys. 2008. PMID: 18464984
Cited by
-
Enantioselective Potassium-Catalyzed Wittig Olefinations.J Am Chem Soc. 2024 Mar 20;146(11):7165-7172. doi: 10.1021/jacs.4c00564. Epub 2024 Mar 7. J Am Chem Soc. 2024. PMID: 38451542 Free PMC article.
-
WDR5 Binding to Histone Serotonylation Is Driven by an Edge-Face Aromatic Interaction with Unexpected Electrostatic Effects.J Am Chem Soc. 2024 Oct 9;146(40):27451-27459. doi: 10.1021/jacs.4c07277. Epub 2024 Sep 25. J Am Chem Soc. 2024. PMID: 39321462
-
The use of hammett constants to understand the non-covalent binding of aromatics.Comput Struct Biotechnol J. 2012 Mar 6;1:e201204004. doi: 10.5936/csbj.201204004. eCollection 2012. Comput Struct Biotechnol J. 2012. PMID: 24688634 Free PMC article. Review.
-
Fragment-Based Approaches to the Development of Mycobacterium tuberculosis CYP121 Inhibitors.J Med Chem. 2016 Apr 14;59(7):3272-302. doi: 10.1021/acs.jmedchem.6b00007. Epub 2016 Mar 22. J Med Chem. 2016. PMID: 27002486 Free PMC article.
-
Inverting Steric Effects: Using "Attractive" Noncovalent Interactions To Direct Silver-Catalyzed Nitrene Transfer.J Am Chem Soc. 2017 Dec 6;139(48):17376-17386. doi: 10.1021/jacs.7b07619. Epub 2017 Nov 20. J Am Chem Soc. 2017. PMID: 29091737 Free PMC article.
References
-
- Birman VB, Jiang H, Guo L, Uffman EW. J. Am. Chem. Soc. 2006;128:6536. - PubMed
-
- Liu J, Brooks NR. Org. Lett. 2002;4:3521. - PubMed
-
- García Ruano JL, Alemán J, Alonso I, Parra A, Marcos V, Aguirre J. Chem. Eur. J. 2007;13:6179. - PubMed
-
- Hunter CA. Chem. Soc. Rev. 1994;23:101.
-
- Claessens CG, Stoddart JF. J. Phys. Org. Chem. 1997;10:254.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
