Dynamics of Microvillus Extension and Tether Formation in Rolling Leukocytes
- PMID: 20046963
- PMCID: PMC2747329
- DOI: 10.1007/s12195-009-0063-9
Dynamics of Microvillus Extension and Tether Formation in Rolling Leukocytes
Abstract
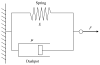
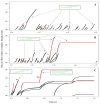
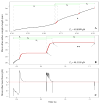
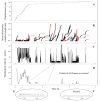
P-selectin glycoprotein ligand-1 (PSGL-1) binding to P-selectin mediates leukocyte rolling under conditions of flow. In human neutrophils, a type of leukocyte belonging to the innate immune system, PSGL-1 molecules are located on the neutrophil's surface ruffles, called microvilli. Each newly formed P-selectin-PSGL-1 bond can become load bearing, imposing on its microvillus a pulling force that deforms the microvillus. Depending on the magnitude of the bond force, a microvillus can be extended, or a thin membrane cylinder (a tether) can be formed at the tip of the microvillus. Here we propose a Kelvin-Voigt viscoelastic material as an improved model for microvillus extension. Using a modified version of our Event-Tracking Model of Adhesion (ETMA), we demonstrate how P-selectin-PSGL-1 load-bearing bonds shape microvillus deformation during neutrophil rolling at low shear (wall shear rate of 50 s(-1), P-selectin site density of 150 molecules μm(-2)). We also discuss the impact of microvillus deformability on neutrophil rolling. We find that the average microvillus extension constitutes 65% of the total microvillus-tether complex extension, and that the rolling neutrophil may never fully rest. A quantitative comparison with the corresponding non-deformable microvilli case supports a concept that the ability of the microvillus to deform stabilizes cell rolling.
Figures


Similar articles
-
Comparison of PSGL-1 microbead and neutrophil rolling: microvillus elongation stabilizes P-selectin bond clusters.Biophys J. 2002 Apr;82(4):1835-47. doi: 10.1016/S0006-3495(02)75534-3. Biophys J. 2002. PMID: 11916843 Free PMC article.
-
Static and dynamic lengths of neutrophil microvilli.Proc Natl Acad Sci U S A. 1998 Jun 9;95(12):6797-802. doi: 10.1073/pnas.95.12.6797. Proc Natl Acad Sci U S A. 1998. PMID: 9618492 Free PMC article.
-
Effect of microvillus deformability on leukocyte adhesion explored using adhesive dynamics simulations.Biophys J. 2005 Jul;89(1):187-200. doi: 10.1529/biophysj.104.054171. Epub 2005 May 6. Biophys J. 2005. PMID: 15879471 Free PMC article.
-
Neutrophil rolling at high shear: flattening, catch bond behavior, tethers and slings.Mol Immunol. 2013 Aug;55(1):59-69. doi: 10.1016/j.molimm.2012.10.025. Epub 2012 Nov 9. Mol Immunol. 2013. PMID: 23141302 Free PMC article. Review.
-
Biomechanics of leukocyte rolling.Biorheology. 2011;48(1):1-35. doi: 10.3233/BIR-2011-0579. Biorheology. 2011. PMID: 21515934 Free PMC article. Review.
Cited by
-
Microfluidics-based side view flow chamber reveals tether-to-sling transition in rolling neutrophils.Sci Rep. 2016 Jun 30;6:28870. doi: 10.1038/srep28870. Sci Rep. 2016. PMID: 27357741 Free PMC article.
-
Cell protrusions and tethers: a unified approach.Biophys J. 2011 Apr 6;100(7):1697-707. doi: 10.1016/j.bpj.2011.02.038. Biophys J. 2011. PMID: 21463583 Free PMC article.
-
Biomechanics of Neutrophil Tethers.Life (Basel). 2021 May 31;11(6):515. doi: 10.3390/life11060515. Life (Basel). 2021. PMID: 34073130 Free PMC article. Review.
-
Leukocyte rolling on P-selectin: a three-dimensional numerical study of the effect of cytoplasmic viscosity.Biophys J. 2012 Apr 18;102(8):1757-66. doi: 10.1016/j.bpj.2012.03.018. Biophys J. 2012. PMID: 22768931 Free PMC article.
-
Ultrastructural analysis of nanogold-labeled cell surface microvilli in liquid by atmospheric scanning electron microscopy and their relevance in cell adhesion.Int J Mol Sci. 2013 Oct 16;14(10):20809-19. doi: 10.3390/ijms141020809. Int J Mol Sci. 2013. PMID: 24135874 Free PMC article.
References
-
- Alon R, Hammer DA, Springer TA. Lifetime of P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 1995;374:539–542. - PubMed
-
- Bell GI. Models for the specific adhesion of cells to cells. Science. 1978;200:618–627. - PubMed
-
- Bruehl RE, Springer TA, Bainton DF. Quantitation of L-selectin distribution on human leukocyte microvilli by immunogold labeling and electron microscopy. J Histochem Cytochem. 1996;44:835–844. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

