Current understanding of SPEM and its standing in the preneoplastic process
- PMID: 20047123
- PMCID: PMC4502916
- DOI: 10.1007/s10120-009-0527-6
Current understanding of SPEM and its standing in the preneoplastic process
Abstract
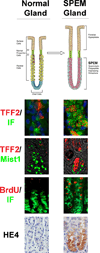
Gastric cancer is the second leading cause of cancer-related death worldwide, but the details of gastric carcinogenesis remain unclear. In humans, two preneoplastic metaplasias are associated with the precancerous stomach: intestinal metaplasia and spasmolytic polypeptide-expressing metaplasia (SPEM). While mouse models of Helicobacter sp. infection have not shown intestinal metaplasia, a number of mouse models lead to the evolution of SPEM. In this review, we summarize increasing data that indicates that SPEM arises in the setting of parietal cell loss, either following acute druginduced oxyntic atrophy or in chronic oxyntic atrophy associated with H. felis infection. Importantly, recent investigations support the origin of SPEM through transdifferentiation from mature chief cells following parietal cell loss. Novel biomarkers of SPEM, such as HE4, hold promise as specific markers of the metaplastic process distinct from normal gastric lineages. Staining with HE4 in humans and other studies in gerbils suggest that SPEM arises initially in the human stomach following parietal cell loss and then further evolves into intestinal metaplasia, likely in association with chronic inflammation. Further studies are needed to broaden our knowledge of metaplasia and early cancer-specific biomarkers that could give insights into both lineage derivation and preneoplasia detection.
Figures

References
-
- Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18–29. - PubMed
-
- Jain RN, Brunkan CS, Chew CS, Samuelson LC. Gene expression profiling of gastrin target genes in parietal cells. Physiol Genomics. 2006 Jan 12;24(2):124–132. - PubMed
-
- Murayama Y, Miyagawa J, Higashiyama S, Kondo S, Yabu M, Kanayama S, et al. Localization of heparin-binding epidermal growth factor-like growth factor (HB-EGF) in human gastric mucosa. Gastroenterology. 1994:A622. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

