The role of metabolites in predicting drug-drug interactions: focus on irreversible cytochrome P450 inhibition
- PMID: 20047147
- PMCID: PMC2898504
The role of metabolites in predicting drug-drug interactions: focus on irreversible cytochrome P450 inhibition
Abstract
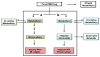
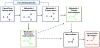
The irreversible inhibition of cytochrome P450 (CYP) enzymes can cause significant drug-drug interactions (DDIs). The formation of metabolites is fundamental for the inactivation of CYP enzymes. Of the 19 CYP enzyme inactivators for which the mechanism of action has been established, 10 have circulating metabolites, which are on the metabolic pathway to inactivation of the CYP enzyme. Because inactivation of CYP enzymes usually requires multiple metabolic steps, the prediction of interactions between metabolites and CYPs in vivo may require complex models and the availability of data generated in vitro from each metabolite. Data discussed in this review suggest that circulating metabolites are more important in CYP inhibition in vivo than has been acknowledged.
Figures


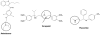
Similar articles
-
Inhibition and induction of cytochrome P450 and the clinical implications.Clin Pharmacokinet. 1998 Nov;35(5):361-90. doi: 10.2165/00003088-199835050-00003. Clin Pharmacokinet. 1998. PMID: 9839089 Review.
-
Mechanism-based inactivation of cytochrome P450 enzymes: chemical mechanisms, structure-activity relationships and relationship to clinical drug-drug interactions and idiosyncratic adverse drug reactions.Curr Drug Metab. 2007 Jun;8(5):407-47. doi: 10.2174/138920007780866807. Curr Drug Metab. 2007. PMID: 17584015 Review.
-
Dynamic and Static Simulations of Fluvoxamine-Perpetrated Drug-Drug Interactions Using Multiple Cytochrome P450 Inhibition Modeling, and Determination of Perpetrator-Specific CYP Isoform Inhibition Constants and Fractional CYP Isoform Contributions to Victim Clearance.J Pharm Sci. 2016 Mar;105(3):1307-17. doi: 10.1016/j.xphs.2015.11.044. Epub 2016 Jan 30. J Pharm Sci. 2016. PMID: 26886336
-
Time-dependent enzyme inactivation: Numerical analyses of in vitro data and prediction of drug-drug interactions.Pharmacol Ther. 2020 Feb;206:107449. doi: 10.1016/j.pharmthera.2019.107449. Epub 2019 Dec 11. Pharmacol Ther. 2020. PMID: 31836452 Free PMC article. Review.
-
Inhibition of cytochrome P450 enzymes and biochemical aspects of mechanism-based inactivation (MBI).Drug Discov Today Technol. 2013 Spring;10(1):e177-89. doi: 10.1016/j.ddtec.2012.09.011. Drug Discov Today Technol. 2013. PMID: 24050247 Review.
Cited by
-
In vitro modulatory effects of flavonoids on human cytochrome P450 2C8 (CYP2C8).Naunyn Schmiedebergs Arch Pharmacol. 2012 May;385(5):495-502. doi: 10.1007/s00210-012-0731-5. Epub 2012 Feb 4. Naunyn Schmiedebergs Arch Pharmacol. 2012. PMID: 22307090
-
A Molecular Aspect in the Regulation of Drug Metabolism: Does PXR-Induced Enzyme Expression Always Lead to Functional Changes in Drug Metabolism?Curr Pharmacol Rep. 2016 Aug;2(4):187-192. doi: 10.1007/s40495-016-0062-1. Epub 2016 May 4. Curr Pharmacol Rep. 2016. PMID: 27795941 Free PMC article.
-
Contributions of UDP-Glucuronosyltransferases to Human Hepatic and Intestinal Metabolism of Ticagrelor and Inhibition of UGTs and Cytochrome P450 Enzymes by Ticagrelor and its Glucuronidated Metabolite.Front Pharmacol. 2021 Oct 14;12:761814. doi: 10.3389/fphar.2021.761814. eCollection 2021. Front Pharmacol. 2021. PMID: 34721047 Free PMC article.
-
Individuals at risk for severe COVID-19 in whom ritonavir-containing therapies are contraindicated or may lead to interactions with concomitant medications: a retrospective analysis of German health insurance claims data.Drugs Context. 2023 Jun 27;12:2023-3-4. doi: 10.7573/dic.2023-3-4. eCollection 2023. Drugs Context. 2023. PMID: 37415918 Free PMC article. Review.
-
Improved Predictions of Drug-Drug Interactions Mediated by Time-Dependent Inhibition of CYP3A.Mol Pharm. 2018 May 7;15(5):1979-1995. doi: 10.1021/acs.molpharmaceut.8b00129. Epub 2018 Apr 10. Mol Pharm. 2018. PMID: 29608318 Free PMC article.
References
-
- He M, Kunze KL, Trager WF. Inhibition of (S)-warfarin metabolism by sulfinpyrazone and its metabolites. Drug Metab Dispos. 1995;23:659–663. - PubMed
-
- He M, Rettie AE, Neal J, Trager WF. Metabolism of sulfinpyrazone sulfide and sulfinpyrazone by human liver microsomes and cDNA-expressed cytochrome P450s. Drug Metab Dispos. 2001;29:701–711. - PubMed
-
- Silverman RB. Mechanism-based Enzyme Inactivation: Chemistry and Enzymology. CRC Press; 1988.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
