NMR mapping of the IFNAR1-EC binding site on IFNalpha2 reveals allosteric changes in the IFNAR2-EC binding site
- PMID: 20047337
- PMCID: PMC2827818
- DOI: 10.1021/bi901313x
NMR mapping of the IFNAR1-EC binding site on IFNalpha2 reveals allosteric changes in the IFNAR2-EC binding site
Abstract
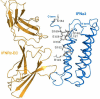
All type I interferons (IFNs) bind to a common cell-surface receptor consisting of two subunits. IFNs initiate intracellular signal transduction cascades by simultaneous interaction with the extracellular domains of its receptor subunits, IFNAR1 and IFNAR2. In this study, we mapped the surface of IFNalpha2 interacting with the extracellular domain of IFNAR1 (IFNAR1-EC) by following changes in or the disappearance of the (1)H-(15)N TROSY-HSQC cross peaks of IFNalpha2 caused by the binding of the extracellular domain of IFNAR1 (IFNAR1-EC) to the binary complex of IFNalpha2 with IFNAR2-EC. The NMR study of the 89 kDa complex was conducted at pH 8 and 308 K using an 800 MHz spectrometer. IFNAR1 binding affected a total of 47 of 165 IFNalpha2 residues contained in two large patches on the face of the protein opposing the binding site for IFNAR2 and in a third patch located on the face containing the IFNAR2 binding site. The first two patches form the IFNAR1 binding site, and one of these matches the IFNAR1 binding site previously identified by site-directed mutagenesis. The third patch partially matches the IFNalpha2 binding site for IFNAR2-EC, indicating allosteric communication between the binding sites for the two receptor subunits.
Figures



References
-
- Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. - PubMed
-
- Novick D, Cohen B, Rubinstein M. The human interferon alpha/beta receptor: characterization and molecular cloning. Cell. 1994;77:391–400. - PubMed
-
- Uze G, Lutfalla G, Mogensen KE. Alpha and beta interferons and their receptor and their friends and relations. J Interferon Cytokine Res. 1995;15:3–26. - PubMed
-
- Piehler J, Schreiber G. Mutational and structural analysis of the binding interface between type I interferons and their receptor Ifnar2. J Mol Biol. 1999;294:223–237. - PubMed
-
- Lamken P, Gavutis M, Peters I, Van der Heyden J, Uze G, Piehler J. Functional cartography of the ectodomain of the type I interferon receptor subunit ifnar1. J Mol Biol. 2005;350:476–488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

