Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice
- PMID: 20047574
- PMCID: PMC2858771
- DOI: 10.1111/j.1474-9726.2009.00545.x
Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice
Abstract
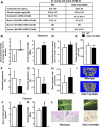
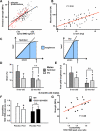
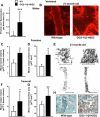
Aging or glucocorticoid excess decrease bone strength more than bone mass in humans and mice, but an explanation for this mismatch remains elusive. We report that aging in C57BL/6 mice was associated with an increase in adrenal production of glucocorticoids as well as bone expression of 11beta-hydroxysteroid dehydrogenase (11beta-HSD) type 1, the enzyme that activates glucocorticoids. Aging also decreased the volume of the bone vasculature and solute transport from the peripheral circulation to the lacunar-canalicular system. The same changes were reproduced by pharmacologic hyperglucocorticoidism. Furthermore, mice in which osteoblasts and osteocytes were shielded from glucocorticoids via cell-specific transgenic expression of 11beta-HSD type 2, the enzyme that inactivates glucocorticoids, were protected from the adverse effects of aging on osteoblast and osteocyte apoptosis, bone formation rate and microarchitecture, crystallinity, vasculature volume, interstitial fluid, and strength. In addition, glucocorticoids suppressed angiogenesis in fetal metatarsals and hypoxia inducible factor-1alpha transcription and vascular endothelial growth factor production in osteoblasts and osteocytes. These results, together with the evidence that dehydration of bone decreases strength, reveal that endogenous glucocorticoids increase skeletal fragility in old age as a result of cell autonomous effects on osteoblasts and osteocytes leading to interconnected decrements in bone angiogenesis, vasculature volume, and osteocyte-lacunar-canalicular fluid.
Keywords: 11β-hydroxysteroid dehydrogenase; Aging; angiogenesis; apoptosis; bone histomorphometry; glucocorticoids; hydraulic support; osteoporosis.
Figures


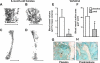
References
-
- Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 2007a;282:27285–27297. - PMC - PubMed
-
- Almeida M, Han L, Martin-Millan M, O'Brien CA, Manolagas SC. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J. Biol. Chem. 2007b;282:27298–27305. - PubMed
-
- Ambrogini E, O'Brien CA, Martin-Millan M, Paik J, DePinho RA, Han L, Warren A, Shelton RS, Qui X, Goellner JJ, Jilka RL, Almeida M, Manolagas SC. Loss or Gain of FoxO Function in Osteoclasts and Osteoblasts Alters the Rate of Apoptosis and BMD in Mice. J. Bone. Min. Res. 2008;23:S70.
-
- Chen W-T, Shih TT, Chen R-C, Lo S-Y, Chou C-T, Lee J-M, Tu H-Y. Vertebral bone marrow perfusion evaluated with dynamic contrast-enhanced MR imaging: significance of aging and sex. Radiology. 2001;220:213–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

