A multicentric observational trial of pegylated liposomal doxorubicin for metastatic breast cancer
- PMID: 20047698
- PMCID: PMC2806246
- DOI: 10.1186/1471-2407-10-2
A multicentric observational trial of pegylated liposomal doxorubicin for metastatic breast cancer
Abstract
Background: Pegylated liposomal doxorubicin (PLD) is active in metastatic breast cancer. This observational study evaluated the efficacy and safety of PLD in patients treated during routine clinical practice.
Methods: Eligible patients had metastatic breast cancer and were treated with PLD according to the dose and schedule determined by their physician as part of routine practice. The primary objectives were to analyze the efficacy and toxicity of PLD therapy.
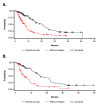
Results: 125 patients were assessable. Median age was 62 years, 78% had performance status 0-1, and 60% had estrogen-receptor-positive disease. PLD treatment was second- or third-line in 69% of patients. Prior anthracyclines (adjuvant or metastatic) had been used in 56% of patients. The majority of patients (79%) received PLD every 4 weeks at a median dose of 40 mg/m2. Overall response rate was 43% in all patients and 34% in those previously treated with anthracyclines. The most common grade 3/4 adverse events were skin toxicity/hand-foot syndrome (6%), and leukopenia (3%).
Conclusions: This observational study supports the activity and tolerability of PLD in metastatic breast cancer as demonstrated in PLD clinical trials.
Figures
References
-
- O'Brien MER, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, Orlandi F, Mellars L, Alland L, Tendler C. CAELYX Breast Cancer Study Group: Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCL (CAELYX™/Doxil®) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440–9. doi: 10.1093/annonc/mdh097. - DOI - PubMed
-
- Al-Batran S, Meerpohl HG, von Minckwitz G A, Kleeberg U, Harbeck N, Lerbs W, Hecker D, Sehouli J, Knuth A, Jager E. Reduced incidence of severe palmar-plantar erythrodysesthesia and mucositis in a prospective multicenter phase II trial with pegylated liposomal doxorubicin at 40 mg/m2 every 4 weeks in previously treated patients with metastatic breast cancer. Oncology. 2006;70:141–6. doi: 10.1159/000093005. - DOI - PubMed
-
- Coleman RE, Biganzoli L, Canney P, Dirix L, Mauriac L, Chollet P, Batter V, Ngalula-Kabanga E, Dittrich C, Piccart M. A randomised phase II study of two different schedules of pegylated liposomal doxorubicin in metastatic breast cancer (EORTC-10993) Eur J Cancer. 2006;42:882–887. doi: 10.1016/j.ejca.2005.12.011. - DOI - PubMed
-
- Al-Batran SE, Bischoff J, von Minckwitz G, Atmaca A, Kleeberg U, Meuthen I, Morack G, Lerbs W, Hecker D, Sehouli J, Knuth A, Jager E. The clinical benefit of pegylated liposomal doxorubicin in patients with metastatic breast cancer previously treated with conventional anthracyclines a multicentre phase II trial. Br J Cancer. 2006;94:1615–20. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

