Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice
- PMID: 20047900
- PMCID: PMC2822632
- DOI: 10.1093/brain/awp309
Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice
Abstract
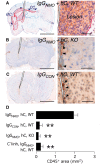
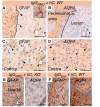
Neuromyelitis optica is an inflammatory demyelinating disease of the central nervous system associated with autoantibodies against the glial water channel protein aquaporin-4. It has recently been reported that immunoglobulin from neuromyelitis optica patients injected peripherally does not cause lesions in naive rats, but only when pre-existing central nervous system inflammation is present. Here, we investigated whether immunoglobulin G from aquaporin-4-autoantibody-positive neuromyelitis optica patients has the potential to damage the central nervous system either alone or in the presence of human complement. Immunoglobulin G from neuromyelitis optica patients did not activate mouse complement and was not pathogenic when injected into mouse brain. However, co-injection of immunoglobulin G from neuromyelitis optica patients with human complement produced neuromyelitis optica-like lesions in mice. Within 12 h of co-injecting immunoglobulin G from neuromyelitis optica patients and human complement, there was a striking loss of aquaporin-4 expression, glial cell oedema, myelin breakdown and axonal injury, but little intra-parenchymal inflammation. At 7 days, there was extensive inflammatory cell infiltration, perivascular deposition of activated complement components, extensive demyelination, loss of aquaporin-4 expression, loss of reactive astrocytes and neuronal cell death. In behavioural studies, mice injected with immunoglobulin G from neuromyelitis optica patients and human complement into the right hemisphere preferentially turned to the right at 7 days. No brain inflammation, demyelination or right-turning behaviour was seen in wild-type mice that received immunoglobulin G from non-neuromyelitis optica patients with human complement, or in aquaporin-4-null mice that received immunoglobulin G from neuromyelitis optica patients with human complement. We conclude that co-injection of immunoglobulin G from neuromyelitis optica patients with human complement reproduces the key histological features of neuromyelitis optica and that aquaporin-4 is necessary and sufficient for immunoglobulin G from neuromyelitis optica patients to exert its effect. In our mouse model, immunoglobulin G from neuromyelitis optica patients does not require pre-existing central nervous system inflammation to produce lesions.
Figures









References
-
- Bradl M, Misu T, Takahashi T, Watanabe M, Mader S, Reindl M, et al. Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann Neurol. 2009 in press. - PubMed
-
- Hinson SR, Pittock SJ, Lucchinetti CF, Roemer SF, Fryer JP, Kryzer TJ, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221–31. - PubMed
-
- Ito S, Mori M, Makino T, Hayakawa S, Kuwabara S. ‘Cloud-like enhancement’ is a magnetic resonance imaging abnormality specific to neuromyelitis optica. Ann Neurol. 2009;66:425–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

