In vitro and in vivo activities of the novel anticytomegalovirus compound AIC246
- PMID: 20047911
- PMCID: PMC2826024
- DOI: 10.1128/AAC.01596-09
In vitro and in vivo activities of the novel anticytomegalovirus compound AIC246
Abstract
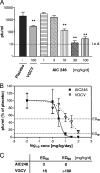
Human cytomegalovirus (HCMV) remains a serious threat for immunocompromised individuals, including transplant recipients and newborns. To date, all drugs licensed for the treatment of HCMV infection and disease target the viral DNA polymerase. Although these drugs are effective, several drawbacks are associated with their use, including toxicity and emergence of drug resistance. Hence, new and improved antivirals with novel molecular targets are urgently needed. Here we report on the antiviral properties of AIC246, a representative of a novel class of low-molecular-weight compounds that is currently undergoing clinical phase II studies. The anti-HCMV activity of AIC246 was evaluated in vitro and in vivo using various cell culture assays and an engineered mouse xenograft model. In addition, antiviral properties of the drug were characterized in comparison to the current gold standard ganciclovir. We demonstrate that AIC246 exhibits excellent in vitro inhibitory activity against HCMV laboratory strains and clinical isolates, retains activity against ganciclovir-resistant viruses, is well tolerated in different cell types (median selectivity index, 18,000), and exerts a potent in vivo efficacy in a mouse xenograft model. Moreover, we show that the antiviral block induced by AIC246 is reversible and the efficacy of the drug is not significantly affected by cell culture variations such as cell type or multiplicity of infection. Finally, initial mode-of-action analyses reveal that AIC246 targets a process in the viral replication cycle that occurs later than DNA synthesis. Thus, AIC246 acts via a mode of action that differs from that of polymerase inhibitors like ganciclovir.
Figures


Similar articles
-
The novel anticytomegalovirus compound AIC246 (Letermovir) inhibits human cytomegalovirus replication through a specific antiviral mechanism that involves the viral terminase.J Virol. 2011 Oct;85(20):10884-93. doi: 10.1128/JVI.05265-11. Epub 2011 Jul 13. J Virol. 2011. PMID: 21752907 Free PMC article.
-
In vitro evaluation of the activities of the novel anticytomegalovirus compound AIC246 (letermovir) against herpesviruses and other human pathogenic viruses.Antimicrob Agents Chemother. 2012 Feb;56(2):1135-7. doi: 10.1128/AAC.05908-11. Epub 2011 Nov 21. Antimicrob Agents Chemother. 2012. PMID: 22106211 Free PMC article.
-
In vitro drug combination studies of Letermovir (AIC246, MK-8228) with approved anti-human cytomegalovirus (HCMV) and anti-HIV compounds in inhibition of HCMV and HIV replication.Antimicrob Agents Chemother. 2015;59(6):3140-8. doi: 10.1128/AAC.00114-15. Epub 2015 Mar 16. Antimicrob Agents Chemother. 2015. PMID: 25779572 Free PMC article.
-
Clinical development of letermovir and maribavir: Overview of human cytomegalovirus drug resistance.Antiviral Res. 2019 Mar;163:91-105. doi: 10.1016/j.antiviral.2019.01.011. Epub 2019 Jan 25. Antiviral Res. 2019. PMID: 30690043 Review.
-
Early inhibitors of human cytomegalovirus: state-of-art and therapeutic perspectives.Pharmacol Ther. 2011 Sep;131(3):309-29. doi: 10.1016/j.pharmthera.2011.04.007. Epub 2011 Apr 28. Pharmacol Ther. 2011. PMID: 21570424 Free PMC article. Review.
Cited by
-
Relative frequency of cytomegalovirus UL56 gene mutations detected in genotypic letermovir resistance testing.Antiviral Res. 2022 Nov;207:105422. doi: 10.1016/j.antiviral.2022.105422. Epub 2022 Sep 25. Antiviral Res. 2022. PMID: 36170912 Free PMC article.
-
Human cytomegalovirus infection and atherothrombosis.J Thromb Thrombolysis. 2012 Feb;33(2):160-72. doi: 10.1007/s11239-011-0662-x. J Thromb Thrombolysis. 2012. PMID: 22161772 Review.
-
Approved Antiviral Drugs over the Past 50 Years.Clin Microbiol Rev. 2016 Jul;29(3):695-747. doi: 10.1128/CMR.00102-15. Clin Microbiol Rev. 2016. PMID: 27281742 Free PMC article. Review.
-
A Native Human Monoclonal Antibody Targeting HCMV gB (AD-2 Site I).Int J Mol Sci. 2018 Dec 11;19(12):3982. doi: 10.3390/ijms19123982. Int J Mol Sci. 2018. PMID: 30544903 Free PMC article. Review.
-
New Developments in the Management of Cytomegalovirus Infection After Transplantation.Drugs. 2018 Jul;78(11):1085-1103. doi: 10.1007/s40265-018-0943-1. Drugs. 2018. PMID: 29961185 Review.
References
-
- Adler, S. P., G. Nigro, and L. Pereira. 2007. Recent advances in the prevention and treatment of congenital cytomegalovirus infections. Semin. Perinatol. 31:10-18. - PubMed
-
- Andrei, G., E. De Clercq, and R. Snoeck. 2008. Novel inhibitors of human CMV. Curr. Opin. Investig. Drugs 9:132-145. - PubMed
-
- Andrei, G., E. De Clercq, and R. Snoeck. 2009. Drug targets in cytomegalovirus infection. Infect. Disord. Drug Targets 9:201-222. - PubMed
-
- Andreoni, M., M. Faircloth, L. Vugler, and W. J. Britt. 1989. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J. Virol. Methods 23:157-167. - PubMed
-
- Biron, K. K. 2006. Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 71:154-163. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

