Activity of dalbavancin against Bacillus anthracis in vitro and in a mouse inhalation anthrax model
- PMID: 20047912
- PMCID: PMC2826002
- DOI: 10.1128/AAC.00820-09
Activity of dalbavancin against Bacillus anthracis in vitro and in a mouse inhalation anthrax model
Abstract
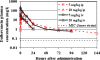
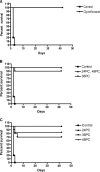
Bacillus anthracis, the causative agent of anthrax, can produce fatal disease when it is inhaled or ingested by humans. Dalbavancin, a novel, semisynthetic lipoglycopeptide, has potent activity, greater than that of vancomycin, against Gram-positive bacteria and a half-life in humans that supports once-weekly dosing. Dalbavancin demonstrated potent in vitro activity against B. anthracis (MIC range, < or =0.03 to 0.5 mg/liter; MIC(50) and MIC(90), 0.06 and 0.25 mg/liter, respectively), which led us to test its efficacy in a murine inhalation anthrax model. The peak concentrations of dalbavancin in mouse plasma after the administration of single intraperitoneal doses of 5 and 20 mg/kg of body weight were 15 and 71 mg/kg, respectively. At 20 mg/kg, the dalbavancin activity was detectable for 6 days after administration (terminal half-life, 53 h), indicating that long intervals between doses were feasible. The mice were challenged with 50 to 100 times the median lethal dose of the Ames strain of B. anthracis, an inoculum that kills untreated animals within 4 days. The efficacy of dalbavancin was 80 to 100%, as determined by the rate of survival at 42 days, when treatment was initiated 24 h postchallenge with regimens of 15 to 120 mg/kg every 36 h (q36h) or 30 to 240 mg/kg every 72 h (q72h). A regimen of ciprofloxacin known to protect 100% of animals was tested in parallel. Delayed dalbavancin treatment (beginning 36 or 48 h postchallenge) with 60 mg/kg q36h or 120 mg/kg q72h still provided 70 to 100% survival. The low MICs and long duration of efficacy in vivo suggest that dalbavancin may have potential as an alternative treatment or for the prophylaxis of B. anthracis infections.
Figures


Similar articles
-
Efficacy of oritavancin in a murine model of Bacillus anthracis spore inhalation anthrax.Antimicrob Agents Chemother. 2008 Sep;52(9):3350-7. doi: 10.1128/AAC.00360-08. Epub 2008 Jul 7. Antimicrob Agents Chemother. 2008. PMID: 18606841 Free PMC article.
-
Pharmacokinetic-pharmacodynamic assessment of faropenem in a lethal murine Bacillus anthracis inhalation postexposure prophylaxis model.Antimicrob Agents Chemother. 2010 May;54(5):1678-83. doi: 10.1128/AAC.00737-08. Epub 2010 Feb 9. Antimicrob Agents Chemother. 2010. PMID: 20145081 Free PMC article.
-
Omadacycline is active in vitro and in vivo against ciprofloxacin-resistant Bacillus anthracis.Antimicrob Agents Chemother. 2024 Sep 4;68(9):e0059524. doi: 10.1128/aac.00595-24. Epub 2024 Aug 12. Antimicrob Agents Chemother. 2024. PMID: 39133023 Free PMC article.
-
Review of dalbavancin, a novel semisynthetic lipoglycopeptide.Expert Opin Investig Drugs. 2007 May;16(5):717-33. doi: 10.1517/13543784.16.5.717. Expert Opin Investig Drugs. 2007. PMID: 17461743 Review.
-
New lipoglycopeptides: a comparative review of dalbavancin, oritavancin and telavancin.Drugs. 2010 May 7;70(7):859-86. doi: 10.2165/11534440-000000000-00000. Drugs. 2010. PMID: 20426497 Review.
Cited by
-
Systematic Review of In Vitro Antimicrobial Susceptibility Testing for Bacillus anthracis, 1947-2019.Clin Infect Dis. 2022 Oct 17;75(Suppl 3):S373-S378. doi: 10.1093/cid/ciac520. Clin Infect Dis. 2022. PMID: 36251548 Free PMC article.
-
New developments in vaccines, inhibitors of anthrax toxins, and antibiotic therapeutics for Bacillus anthracis.Curr Med Chem. 2011;18(33):5083-94. doi: 10.2174/092986711797636036. Curr Med Chem. 2011. PMID: 22050756 Free PMC article. Review.
-
Lipoglycopeptide Antibacterial Agents in Gram-Positive Infections: A Comparative Review.Drugs. 2015 Dec;75(18):2073-95. doi: 10.1007/s40265-015-0505-8. Drugs. 2015. PMID: 26603874 Review.
-
Postexposure Prophylaxis and Treatment of Bacillus anthracis Infections: A Systematic Review and Meta-analyses of Animal Models, 1947-2019.Clin Infect Dis. 2022 Oct 17;75(Suppl 3):S379-S391. doi: 10.1093/cid/ciac591. Clin Infect Dis. 2022. PMID: 36251546 Free PMC article.
-
Evaluation of liposomal ciprofloxacin formulations in a murine model of anthrax.PLoS One. 2020 Jan 24;15(1):e0228162. doi: 10.1371/journal.pone.0228162. eCollection 2020. PLoS One. 2020. PMID: 31978152 Free PMC article.
References
-
- Bradaric, N., and V. Punda-Polic. 1992. Cutaneous anthrax due to penicillin-resistant Bacillus anthracis transmitted by an insect bite. Lancet 340:306-307. - PubMed
-
- Brook, I., T. B. Elliott, H. I. Pryor II, T. E. Sautter, B. T. Gnade, J. H. Thakar, and G. B. Knudson. 2001. In vitro resistance of Bacillus anthracis Sterne to doxycycline, macrolides and quinolones. Int. J. Antimicrob. Agents 18:559-562. - PubMed
-
- Buckwalter, M., and J. A. Dowell. 2005. Population pharmacokinetic analysis of dalbavancin, a novel lipoglycopeptide. J. Clin. Pharmacol. 45:1279-1287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

