Multiplex SILAC analysis of a cellular TDP-43 proteinopathy model reveals protein inclusions associated with SUMOylation and diverse polyubiquitin chains
- PMID: 20047951
- PMCID: PMC2860236
- DOI: 10.1074/mcp.M800390-MCP200
Multiplex SILAC analysis of a cellular TDP-43 proteinopathy model reveals protein inclusions associated with SUMOylation and diverse polyubiquitin chains
Abstract
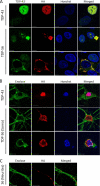
Transactive response (TAR) DNA-binding protein 43 (TDP-43) is a major protein component within ubiquitin-positive inclusions of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Although TDP-43 is a nuclear DNA/RNA-binding protein, in pathological conditions, TDP-43 has been reported to redistribute to the cytoplasm where it is cleaved and forms insoluble, ubiquitinated, and phosphorylated inclusions. Here we present a cellular model in which full-length human TDP-43 or a splicing isoform (TDP-S6) that lacks the C terminus is overexpressed in a human cell line and mouse primary neurons. Whereas recombinant and endogenous TDP-43 was primarily localized in the nucleus, the shorter TDP-S6 formed highly insoluble cytoplasmic and nuclear inclusions reminiscent of disease-specific pathology. Western blot analysis of detergent-insoluble extracts showed an increase in high molecular weight immunoreactive species for TDP-S6 compared with TDP-43, consistent with ubiquitination or ubiquitin-like modifications. We used a multiplex stable isotope labeling with amino acids in cell culture approach to compare the detergent-insoluble proteome from mock-, TDP-43-, and TDP-S6-transfected cells. TDP-S6 overexpression caused a concomitant increase in both ubiquitin (Ub) and the small Ub-like modifier-2/3 (SUMO-2/3) within the insoluble proteome. Similarly, full-length TDP-43 overexpression also resulted in the elevation of SUMO-2/3. Immunofluorescence showed strong co-localization of endogenous Ub with both cytoplasmic and nuclear TDP-S6 inclusions, whereas SUMO-2/3 was co-localized mainly with the nuclear inclusions. Quantitative mass spectrometry further revealed that mixed Lys-48 and Lys-63 polyUb linkages were associated with the TDP insoluble fractions. Together our data indicate that expression of a TDP-43 splice variant lacking a C terminus recapitulates many of the cellular and biochemical features associated with disease pathology and that the interplay of ubiquitination and SUMOylation may have an important role in TDP-43 regulation.
Figures


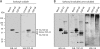




Similar articles
-
Coaggregation of RNA-binding proteins in a model of TDP-43 proteinopathy with selective RGG motif methylation and a role for RRM1 ubiquitination.PLoS One. 2012;7(6):e38658. doi: 10.1371/journal.pone.0038658. Epub 2012 Jun 21. PLoS One. 2012. PMID: 22761693 Free PMC article.
-
Quantitative analysis of the detergent-insoluble brain proteome in frontotemporal lobar degeneration using SILAC internal standards.J Proteome Res. 2012 May 4;11(5):2721-38. doi: 10.1021/pr2010814. Epub 2012 Apr 4. J Proteome Res. 2012. PMID: 22416763 Free PMC article.
-
Ubiquitinated, p62 immunopositive cerebellar cortical neuronal inclusions are evident across the spectrum of TDP-43 proteinopathies but are only rarely additionally immunopositive for phosphorylation-dependent TDP-43.Neuropathology. 2011 Jun;31(3):239-49. doi: 10.1111/j.1440-1789.2010.01171.x. Epub 2010 Dec 1. Neuropathology. 2011. PMID: 21118398
-
[The molecular mechanisms of intracellular TDP-43 aggregates].Brain Nerve. 2009 Nov;61(11):1292-300. Brain Nerve. 2009. PMID: 19938686 Review. Japanese.
-
Rodent models of TDP-43 proteinopathy: investigating the mechanisms of TDP-43-mediated neurodegeneration.J Mol Neurosci. 2011 Nov;45(3):486-99. doi: 10.1007/s12031-011-9610-7. Epub 2011 Aug 3. J Mol Neurosci. 2011. PMID: 21811811 Free PMC article. Review.
Cited by
-
Global quantitative analysis of the human brain proteome and phosphoproteome in Alzheimer's disease.Sci Data. 2020 Sep 28;7(1):315. doi: 10.1038/s41597-020-00650-8. Sci Data. 2020. PMID: 32985496 Free PMC article.
-
SUMO3 modification accelerates the aggregation of ALS-linked SOD1 mutants.PLoS One. 2014 Jun 27;9(6):e101080. doi: 10.1371/journal.pone.0101080. eCollection 2014. PLoS One. 2014. PMID: 24971881 Free PMC article.
-
TDP43 autoregulation gives rise to dominant negative isoforms that are tightly controlled by transcriptional and post-translational mechanisms.Cell Rep. 2025 Jan 28;44(1):115113. doi: 10.1016/j.celrep.2024.115113. Epub 2025 Jan 9. Cell Rep. 2025. PMID: 39792557 Free PMC article.
-
SUMO2/3 conjugation of TDP-43 protects against aggregation.Sci Adv. 2025 Feb 21;11(8):eadq2475. doi: 10.1126/sciadv.adq2475. Epub 2025 Feb 21. Sci Adv. 2025. PMID: 39982984 Free PMC article.
-
Proteomic analysis of hippocampal dentate granule cells in frontotemporal lobar degeneration: application of laser capture technology.Front Neurol. 2011 Apr 25;2:24. doi: 10.3389/fneur.2011.00024. eCollection 2011. Front Neurol. 2011. PMID: 21577247 Free PMC article.
References
-
- Neary D., Snowden J., Mann D. (2005) Frontotemporal dementia. Lancet Neurol 4, 771–780 - PubMed
-
- Ross C. A., Poirier M. A. (2004) Protein aggregation and neurodegenerative disease. Nat. Med 10, (suppl.) S10–S17 - PubMed
-
- Taylor J. P., Hardy J., Fischbeck K. H. (2002) Toxic proteins in neurodegenerative disease. Science 296, 1991–1995 - PubMed
-
- Neumann M., Sampathu D. M., Kwong L. K., Truax A. C., Micsenyi M. C., Chou T. T., Bruce J., Schuck T., Grossman M., Clark C. M., McCluskey L. F., Miller B. L., Masliah E., Mackenzie I. R., Feldman H., Feiden W., Kretzschmar H. A., Trojanowski J. Q., Lee V. M. (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

