The ribosome assembly factor Nep1 responsible for Bowen-Conradi syndrome is a pseudouridine-N1-specific methyltransferase
- PMID: 20047967
- PMCID: PMC2853112
- DOI: 10.1093/nar/gkp1189
The ribosome assembly factor Nep1 responsible for Bowen-Conradi syndrome is a pseudouridine-N1-specific methyltransferase
Abstract
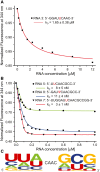
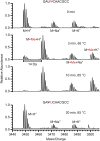
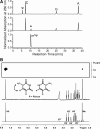
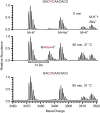
Nep1 (Emg1) is a highly conserved nucleolar protein with an essential function in ribosome biogenesis. A mutation in the human Nep1 homolog causes Bowen-Conradi syndrome-a severe developmental disorder. Structures of Nep1 revealed a dimer with a fold similar to the SPOUT-class of RNA-methyltransferases suggesting that Nep1 acts as a methyltransferase in ribosome biogenesis. The target for this putative methyltransferase activity has not been identified yet. We characterized the RNA-binding specificity of Methanocaldococcus jannaschii Nep1 by fluorescence- and NMR-spectroscopy as well as by yeast three-hybrid screening. Nep1 binds with high affinity to short RNA oligonucleotides corresponding to nt 910-921 of M. jannaschii 16S rRNA through a highly conserved basic surface cleft along the dimer interface. Nep1 only methylates RNAs containing a pseudouridine at a position corresponding to a previously identified hypermodified N1-methyl-N3-(3-amino-3-carboxypropyl) pseudouridine (m1acp3-Psi) in eukaryotic 18S rRNAs. Analysis of the methylated nucleoside by MALDI-mass spectrometry, HPLC and NMR shows that the methyl group is transferred to the N1 of the pseudouridine. Thus, Nep1 is the first identified example of an N1-specific pseudouridine methyltransferase. This enzymatic activity is also conserved in human Nep1 suggesting that Nep1 is the methyltransferase in the biosynthesis of m1acp3-Psi in eukaryotic 18S rRNAs.
Figures

Similar articles
-
The Bowen-Conradi syndrome protein Nep1 (Emg1) has a dual role in eukaryotic ribosome biogenesis, as an essential assembly factor and in the methylation of Ψ1191 in yeast 18S rRNA.Nucleic Acids Res. 2011 Mar;39(4):1526-37. doi: 10.1093/nar/gkq931. Epub 2010 Oct 23. Nucleic Acids Res. 2011. PMID: 20972225 Free PMC article.
-
Structural insight into the functional mechanism of Nep1/Emg1 N1-specific pseudouridine methyltransferase in ribosome biogenesis.Nucleic Acids Res. 2011 Mar;39(6):2445-57. doi: 10.1093/nar/gkq1131. Epub 2010 Nov 17. Nucleic Acids Res. 2011. PMID: 21087996 Free PMC article.
-
Structural analysis of the ribosome assembly factor Nep1, an N1-specific pseudouridine methyltransferase, reveals mechanistic insights.FEBS J. 2025 May;292(9):2338-2358. doi: 10.1111/febs.70005. Epub 2025 Feb 7. FEBS J. 2025. PMID: 39918246
-
Pseudouridines and pseudouridine synthases of the ribosome.Cold Spring Harb Symp Quant Biol. 2001;66:147-59. doi: 10.1101/sqb.2001.66.147. Cold Spring Harb Symp Quant Biol. 2001. PMID: 12762017 Review.
-
Pseudouridine and O2'-methylated nucleosides. Significance of their selective occurrence in rRNA domains that function in ribosome-catalyzed synthesis of the peptide bonds in proteins.Biochimie. 1995;77(1-2):7-15. doi: 10.1016/0300-9084(96)88098-9. Biochimie. 1995. PMID: 7599278 Review.
Cited by
-
Molecular basis of the human ribosomopathy Shwachman-Diamond syndrome.Adv Biol Regul. 2018 Jan;67:109-127. doi: 10.1016/j.jbior.2017.09.002. Epub 2017 Sep 6. Adv Biol Regul. 2018. PMID: 28942353 Free PMC article. Review.
-
Quality control ensures fidelity in ribosome assembly and cellular health.J Cell Biol. 2023 Apr 3;222(4):e202209115. doi: 10.1083/jcb.202209115. Epub 2023 Feb 15. J Cell Biol. 2023. PMID: 36790396 Free PMC article. Review.
-
Human diseases of the SSU processome.Biochim Biophys Acta. 2014 Jun;1842(6):758-64. doi: 10.1016/j.bbadis.2013.11.004. Epub 2013 Nov 12. Biochim Biophys Acta. 2014. PMID: 24240090 Free PMC article. Review.
-
Mouse Models of Rare Craniofacial Disorders.Curr Top Dev Biol. 2015;115:413-58. doi: 10.1016/bs.ctdb.2015.07.011. Curr Top Dev Biol. 2015. PMID: 26589934 Free PMC article. Review.
-
The antiproliferative effect of FGF2 in K-Ras-driven tumor cells involves modulation of rRNA and the nucleolus.J Cell Sci. 2023 Nov 15;136(22):jcs260989. doi: 10.1242/jcs.260989. Epub 2023 Nov 30. J Cell Sci. 2023. PMID: 37921359 Free PMC article.
References
-
- Decatur WA, Fournier MJ. rRNA modifications and ribosome function. Trends Biochem. Sci. 2002;27:344–351. - PubMed
-
- King TH, Liu B, McCully RR, Fournier MJ. Ribosome structure and activity are altered in cells lacking snoRNPs that form pseudouridines in the peptidyl transferase center. Mol. Cell. 2003;11:425–435. - PubMed
-
- Caldas T, Binet E, Bouloc P, Costa A, Desgres J, Richarme G. The FtsJ/RrmJ heat shock protein of Escherichia coli is a 23 S ribosomal RNA methyltransferase. J. Biol. Chem. 2000;275:16414–16419. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

