Mph1 requires mismatch repair-independent and -dependent functions of MutSalpha to regulate crossover formation during homologous recombination repair
- PMID: 20047969
- PMCID: PMC2847250
- DOI: 10.1093/nar/gkp1199
Mph1 requires mismatch repair-independent and -dependent functions of MutSalpha to regulate crossover formation during homologous recombination repair
Abstract
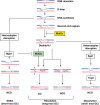
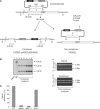
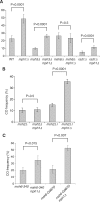
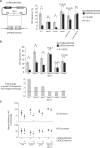
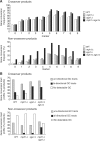
In budding yeast the DNA helicase Mph1 prevents genome rearrangements during ectopic homologous recombination (HR) by suppressing the formation of crossovers (COs). Here we show that during ectopic HR repair, the anti-CO function of Mph1 is intricately associated with the mismatch repair (MMR) factor, MutSalpha. In particular, during HR repair using a completely homologous substrate, we reveal an MMR-independent function of MutSalpha in generating COs that is specifically antagonized by Mph1, but not Sgs1. In contrast, both Mph1 and MutSalpha are required to efficiently suppress COs in the presence of a homeologous substrate. Mph1 acts redundantly with Sgs1 in this respect since mph1Delta sgs1Delta double mutant cells pheno-copy MutSalpha mutants and completely fail to discriminate homologous and homeologous sequences during HR repair. However, this defect of mph1Delta sgs1Delta cells is not due to an inability to carry out MMR but rather is accompanied by elevated levels of gene conversion (GC) and bi-directional GC tracts specifically in non-crossover products. Models describing how Mph1, MutSalpha and Sgs1 act in concert to suppress genome rearrangements during ectopic HR repair are discussed.
Figures
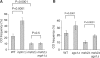

Similar articles
-
Mph1 and Mus81-Mms4 prevent aberrant processing of mitotic recombination intermediates.Mol Cell. 2013 Oct 10;52(1):63-74. doi: 10.1016/j.molcel.2013.09.007. Mol Cell. 2013. PMID: 24119400 Free PMC article.
-
Components of a Fanconi-like pathway control Pso2-independent DNA interstrand crosslink repair in yeast.PLoS Genet. 2012;8(8):e1002884. doi: 10.1371/journal.pgen.1002884. Epub 2012 Aug 9. PLoS Genet. 2012. PMID: 22912599 Free PMC article.
-
Homologous recombination-dependent rescue of deficiency in the structural maintenance of chromosomes (Smc) 5/6 complex.J Biol Chem. 2011 Feb 18;286(7):5119-25. doi: 10.1074/jbc.M110.201608. Epub 2010 Dec 7. J Biol Chem. 2011. PMID: 21138837 Free PMC article.
-
Sgs1 and Mph1 Helicases Enforce the Recombination Execution Checkpoint During DNA Double-Strand Break Repair in Saccharomyces cerevisiae.Genetics. 2016 Jun;203(2):667-75. doi: 10.1534/genetics.115.184317. Epub 2016 Apr 13. Genetics. 2016. PMID: 27075725 Free PMC article.
-
Heteroduplex DNA position defines the roles of the Sgs1, Srs2, and Mph1 helicases in promoting distinct recombination outcomes.PLoS Genet. 2013;9(3):e1003340. doi: 10.1371/journal.pgen.1003340. Epub 2013 Mar 14. PLoS Genet. 2013. PMID: 23516370 Free PMC article.
Cited by
-
Molecular structures of crossover and noncrossover intermediates during gap repair in yeast: implications for recombination.Mol Cell. 2010 Apr 23;38(2):211-22. doi: 10.1016/j.molcel.2010.02.028. Mol Cell. 2010. PMID: 20417600 Free PMC article.
-
Factors that influence bidirectional long-tract homozygosis due to double-strand break repair in Candida albicans.Genetics. 2021 May 17;218(1):iyab028. doi: 10.1093/genetics/iyab028. Genetics. 2021. PMID: 33705548 Free PMC article.
-
The roles of the Saccharomyces cerevisiae RecQ helicase SGS1 in meiotic genome surveillance.PLoS One. 2010 Nov 9;5(11):e15380. doi: 10.1371/journal.pone.0015380. PLoS One. 2010. PMID: 21085703 Free PMC article.
-
Mph1 and Mus81-Mms4 prevent aberrant processing of mitotic recombination intermediates.Mol Cell. 2013 Oct 10;52(1):63-74. doi: 10.1016/j.molcel.2013.09.007. Mol Cell. 2013. PMID: 24119400 Free PMC article.
-
Collaborations between chromatin and nuclear architecture to optimize DNA repair fidelity.DNA Repair (Amst). 2021 Jan;97:103018. doi: 10.1016/j.dnarep.2020.103018. Epub 2020 Nov 22. DNA Repair (Amst). 2021. PMID: 33285474 Free PMC article. Review.
References
-
- Wu L, Hickson ID. The Bloom’s syndrome helicase suppresses crossing over during homologous recombination. Nature. 2003;426:870–874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

