Pseudomonas aeruginosa alginate promotes Burkholderia cenocepacia persistence in cystic fibrosis transmembrane conductance regulator knockout mice
- PMID: 20048042
- PMCID: PMC2825924
- DOI: 10.1128/IAI.01192-09
Pseudomonas aeruginosa alginate promotes Burkholderia cenocepacia persistence in cystic fibrosis transmembrane conductance regulator knockout mice
Abstract
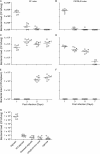
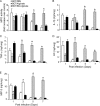
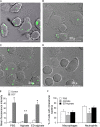
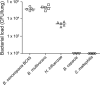
Pseudomonas aeruginosa, a major respiratory pathogen in cystic fibrosis (CF) patients, facilitates infection by other opportunistic pathogens. Burkholderia cenocepacia, which normally infects adolescent patients, encounters alginate elaborated by mucoid P. aeruginosa. To determine whether P. aeruginosa alginate facilitates B. cenocepacia infection in mice, cystic fibrosis transmembrane conductance regulator knockout mice were infected with B. cenocepacia strain BC7 suspended in either phosphate-buffered saline (BC7/PBS) or P. aeruginosa alginate (BC7/alginate), and the pulmonary bacterial load and inflammation were monitored. Mice infected with BC7/PBS cleared all of the bacteria within 3 days, and inflammation was resolved by day 5. In contrast, mice infected with BC7/alginate showed persistence of bacteria and increased cytokine levels for up to 7 days. Histological examination of the lungs indicated that there was moderate to severe inflammation and pneumonic consolidation in isolated areas at 5 and 7 days postinfection in the BC7/alginate group. Further, alginate decreased phagocytosis of B. cenocepacia by professional phagocytes both in vivo and in vitro. P. aeruginosa alginate also reduced the proinflammatory responses of CF airway epithelial cells and alveolar macrophages to B. cenocepacia infection. The observed effects are specific to P. aeruginosa alginate, because enzymatically degraded alginate or other polyuronic acids did not facilitate bacterial persistence. These observations suggest that P. aeruginosa alginate may facilitate B. cenocepacia infection by interfering with host innate defense mechanisms.
Figures

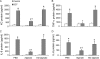

References
-
- Cabral, D. A., B. A. Loh, and D. P. Speert. 1987. Mucoid Pseudomonas aeruginosa resists nonopsonic phagocytosis by human neutrophils and macrophages. Pediatr. Res. 22:429-431. - PubMed
-
- Cesaretti, M., E. Luppi, F. Maccari, and N. Volpi. 2003. A 96-well assay for uronic acid carbazole reaction. Carbohydr. Polym. 54:59-61.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

