Cryptococcus neoformans variants generated by phenotypic switching differ in virulence through effects on macrophage activation
- PMID: 20048044
- PMCID: PMC2825907
- DOI: 10.1128/IAI.01049-09
Cryptococcus neoformans variants generated by phenotypic switching differ in virulence through effects on macrophage activation
Abstract
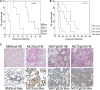
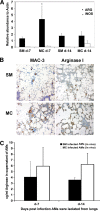
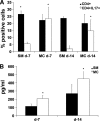
Macrophages have a central role in the pathogenesis of cryptococcosis since they are an important line of defense, serve as a site for fungal replication, and also can contribute to tissue damage. The objective of this study was to investigate the interaction of macrophages with cells from smooth-colony variants (SM) and mucoid-colony variants (MC) arising from phenotypic switching of Cryptococcus neoformans. Alveolar macrophages (AMs) isolated from SM- and MC-infected mice exhibited differences in gene and surface expression of PD-L1, PD-L2, and major histocompatibility class II (MHC-II). PD-L1 and PD-L2 are the ligands for PD1 and are differentially regulated in Th1- and Th2-type cells. In addition, macrophage activation in SM- and MC-infected mice was characterized as alternatively activated. Flow cytometric and cytokine analysis demonstrated that MC infection was associated with the emergence of Th17 cells and higher levels of interleukin-17 (IL-17) in lung tissue, which were reduced by AM depletion. In conclusion, our results indicate that macrophages play a significant role in maintaining damage-promoting inflammation in the lung during MC infection, which ultimately results in death.
Figures



Similar articles
-
Phenotypic switching in Cryptococcus neoformans contributes to virulence by changing the immunological host response.Infect Immun. 2008 Sep;76(9):4322-31. doi: 10.1128/IAI.00529-08. Epub 2008 Jun 30. Infect Immun. 2008. PMID: 18591227 Free PMC article.
-
Cryptococcus neoformans and Cryptococcus gattii clinical isolates from Thailand display diverse phenotypic interactions with macrophages.Virulence. 2019 Dec;10(1):26-36. doi: 10.1080/21505594.2018.1556150. Virulence. 2019. PMID: 30520685 Free PMC article.
-
Phenotypic switching of Cryptococcus neoformans can influence the outcome of the human immune response.Cell Microbiol. 2003 Aug;5(8):513-22. doi: 10.1046/j.1462-5822.2003.00297.x. Cell Microbiol. 2003. PMID: 12864811
-
Phenotypic switching and its implications for the pathogenesis of Cryptococcus neoformans.FEMS Yeast Res. 2006 Jun;6(4):480-8. doi: 10.1111/j.1567-1364.2006.00039.x. FEMS Yeast Res. 2006. PMID: 16696644 Free PMC article. Review.
-
Phenotypic switching in Cryptococcus neoformans.Microbiology (Reading). 2006 Jan;152(Pt 1):3-9. doi: 10.1099/mic.0.28451-0. Microbiology (Reading). 2006. PMID: 16385110 Free PMC article. Review.
Cited by
-
Cryptococcal interactions with the host immune system.Eukaryot Cell. 2010 Jun;9(6):835-46. doi: 10.1128/EC.00039-10. Epub 2010 Apr 9. Eukaryot Cell. 2010. PMID: 20382758 Free PMC article. Review.
-
IL-23 dampens the allergic response to Cryptococcus neoformans through IL-17-independent and -dependent mechanisms.Am J Pathol. 2012 Apr;180(4):1547-59. doi: 10.1016/j.ajpath.2011.12.038. Epub 2012 Feb 16. Am J Pathol. 2012. PMID: 22342846 Free PMC article.
-
Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box.Nat Rev Microbiol. 2011 Mar;9(3):193-203. doi: 10.1038/nrmicro2522. Nat Rev Microbiol. 2011. PMID: 21326274 Free PMC article. Review.
-
Early or late IL-10 blockade enhances Th1 and Th17 effector responses and promotes fungal clearance in mice with cryptococcal lung infection.J Immunol. 2014 Oct 15;193(8):4107-16. doi: 10.4049/jimmunol.1400650. Epub 2014 Sep 15. J Immunol. 2014. PMID: 25225664 Free PMC article.
-
Pathogenicity and virulence of Cryptococcus neoformans from an environmental perspective.Virulence. 2025 Dec;16(1):2547090. doi: 10.1080/21505594.2025.2547090. Epub 2025 Aug 14. Virulence. 2025. PMID: 40810603 Free PMC article. Review.
References
-
- Alvarez, M., and A. Casadevall. 2006. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr. Biol. 16:2161-2165. - PubMed
-
- Arora, S., Y. Hernandez, J. R. Erb-Downward, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2005. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J. Immunol. 174:6346-6356. - PubMed
-
- Baetselier, P. D., B. Namangala, W. Noel, L. Brys, E. Pays, and A. Beschin. 2001. Alternative versus classical macrophage activation during experimental African trypanosomosis. Int. J. Parasitol. 31:575-587. - PubMed
-
- Bronte, V., and P. Zanovello. 2005. Regulation of immune responses by l-arginine metabolism. Nat. Rev. Immunol. 5:641-654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

