Pore formation triggered by Legionella spp. is an Nlrc4 inflammasome-dependent host cell response that precedes pyroptosis
- PMID: 20048047
- PMCID: PMC2825914
- DOI: 10.1128/IAI.00905-09
Pore formation triggered by Legionella spp. is an Nlrc4 inflammasome-dependent host cell response that precedes pyroptosis
Abstract
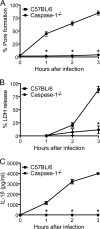
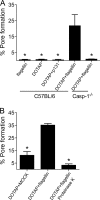
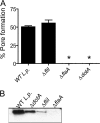
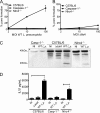
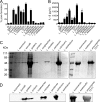
Legionella pneumophila, the etiological agent of Legionnaires disease, is known to trigger pore formation in bone marrow-derived macrophages (BMMs) by mechanisms dependent on the type IVB secretion system known as Dot/Icm. Here, we used several mutants of L. pneumophila in combination with knockout mice to assess the host and bacterial factors involved in pore formation in BMMs. We found that regardless of Dot/Icm activity, pore formation does not occur in BMMs deficient in caspase-1 and Nlrc4/Ipaf. Pore formation was temporally associated with interleukin-1beta secretion and preceded host cell lysis and pyroptosis. Pore-forming ability was dependent on bacterial Dot/Icm but independent of several effector proteins, multiplication, and de novo protein synthesis. Flagellin, which is known to trigger the Nlrc4 inflammasome, was required for pore formation as flaA mutant bacteria failed to induce cell permeabilization. Accordingly, transfection of purified flagellin was sufficient to trigger pore formation independent of infection. By using 11 different Legionella species, we found robust pore formation in response to L. micdadei, L. bozemanii, L. gratiana, L. jordanis, and L. rubrilucens, and this trait correlated with flagellin expression by these species. Together, the results suggest that pore formation is neither L. pneumophila specific nor the result of membrane damage induced by Dot/Icm activity; instead, it is a highly coordinated host cell response dependent on host Nlrc4 and caspase-1 and on bacterial flagellin and type IV secretion system.
Figures
Similar articles
-
Caspase-1 but Not Caspase-11 Is Required for NLRC4-Mediated Pyroptosis and Restriction of Infection by Flagellated Legionella Species in Mouse Macrophages and In Vivo.J Immunol. 2015 Sep 1;195(5):2303-11. doi: 10.4049/jimmunol.1501223. Epub 2015 Jul 31. J Immunol. 2015. PMID: 26232428
-
Activation of NLRC4 by flagellated bacteria triggers caspase-1-dependent and -independent responses to restrict Legionella pneumophila replication in macrophages and in vivo.J Immunol. 2011 Dec 15;187(12):6447-55. doi: 10.4049/jimmunol.1003784. Epub 2011 Nov 11. J Immunol. 2011. PMID: 22079982
-
Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila.Proc Natl Acad Sci U S A. 2013 Jan 29;110(5):1851-6. doi: 10.1073/pnas.1211521110. Epub 2013 Jan 10. Proc Natl Acad Sci U S A. 2013. PMID: 23307811 Free PMC article.
-
Inflammasome Recognition and Regulation of the Legionella Flagellum.Curr Top Microbiol Immunol. 2016;397:161-81. doi: 10.1007/978-3-319-41171-2_8. Curr Top Microbiol Immunol. 2016. PMID: 27460809 Review.
-
Nlrc4/Ipaf/CLAN/CARD12: more than a flagellin sensor.Int J Biochem Cell Biol. 2010 Jun;42(6):789-91. doi: 10.1016/j.biocel.2010.01.003. Epub 2010 Jan 11. Int J Biochem Cell Biol. 2010. PMID: 20067841 Free PMC article. Review.
Cited by
-
Inflammasome-mediated cell death in response to bacterial pathogens that access the host cell cytosol: lessons from legionella pneumophila.Front Cell Infect Microbiol. 2013 Dec 27;3:111. doi: 10.3389/fcimb.2013.00111. Front Cell Infect Microbiol. 2013. PMID: 24409420 Free PMC article. Review.
-
São Paulo School of Advanced Sciences on Vaccines: an overview.J Venom Anim Toxins Incl Trop Dis. 2020 Apr 6;26:e20190061. doi: 10.1590/1678-9199-JVATITD-2019-0061. J Venom Anim Toxins Incl Trop Dis. 2020. PMID: 32362926 Free PMC article. Review.
-
Induction of inflammasome-dependent pyroptosis by carbon black nanoparticles.J Biol Chem. 2011 Jun 17;286(24):21844-52. doi: 10.1074/jbc.M111.238519. Epub 2011 Apr 27. J Biol Chem. 2011. PMID: 21525001 Free PMC article.
-
The intersection of cell death and inflammasome activation.Cell Mol Life Sci. 2016 Jun;73(11-12):2349-67. doi: 10.1007/s00018-016-2205-2. Epub 2016 Apr 11. Cell Mol Life Sci. 2016. PMID: 27066895 Free PMC article. Review.
-
Maternal high-fat diet exaggerates diet-induced insulin resistance in adult offspring by enhancing inflammasome activation through noncanonical pathway of caspase-11.Mol Metab. 2020 Jul;37:100988. doi: 10.1016/j.molmet.2020.100988. Epub 2020 Apr 6. Mol Metab. 2020. PMID: 32272237 Free PMC article.
References
-
- Akhter, A., M. A. Gavrilin, L. Frantz, S. Washington, C. Ditty, D. Limoli, C. Day, A. Sarkar, C. Newland, J. Butchar, C. B. Marsh, M. D. Wewers, S. Tridandapani, T. D. Kanneganti, and A. O. Amer. 2009. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 5:e1000361. - PMC - PubMed
-
- Amer, A., L. Franchi, T. D. Kanneganti, M. Body-Malapel, N. Ozoren, G. Brady, S. Meshinchi, R. Jagirdar, A. Gewirtz, S. Akira, and G. Nunez. 2006. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J. Biol. Chem. 281:35217-35223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

