Sterile alpha motif domain-mediated self-association plays an essential role in modulating the activity of the Drosophila ETS family transcriptional repressor Yan
- PMID: 20048052
- PMCID: PMC2820895
- DOI: 10.1128/MCB.01225-09
Sterile alpha motif domain-mediated self-association plays an essential role in modulating the activity of the Drosophila ETS family transcriptional repressor Yan
Abstract
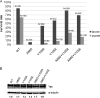
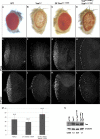
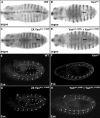
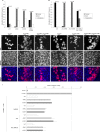
The ETS family transcriptional repressor Yan is an important downstream target and effector of the receptor tyrosine kinase (RTK) signaling pathway in Drosophila melanogaster. Structural and biochemical studies have shown that the N-terminal sterile alpha motif (SAM) of Yan is able to self associate to form a helical polymeric structure in vitro, although the extent and functional significance of self-association of full-length Yan remain unclear. In this study, we demonstrated that full-length Yan self associates via its SAM domain to form higher-order complexes in living cells. Introduction of SAM domain missense mutations that restrict Yan to a monomeric state reduces Yan's transcriptional repression activity and impairs its function during embryonic and retinal development. Coexpression of combinations of SAM domain mutations that permit the formation of Yan dimers, but not higher-order oligomers, increases activity relative to that of monomeric Yan, but not to the level obtained with wild-type Yan. Mechanistically, self-association directly promotes transcriptional repression of target genes independent of its role in limiting mitogen-activated protein kinase (MAPK)-mediated phosphorylation and nuclear export of Yan. Thus, we propose that the formation of higher-order Yan oligomers contributes to proper repression of target gene expression and RTK signaling output in developing tissues.
Figures



Similar articles
-
The relationship between long-range chromatin occupancy and polymerization of the Drosophila ETS family transcriptional repressor Yan.Genetics. 2013 Feb;193(2):633-49. doi: 10.1534/genetics.112.146647. Epub 2012 Nov 19. Genetics. 2013. PMID: 23172856 Free PMC article.
-
Chromatin occupancy patterns of the ETS repressor Yan: a mechanism for buffering gene expression against noise?Fly (Austin). 2013 Apr-Jun;7(2):92-8. doi: 10.4161/fly.24162. Epub 2013 Apr 1. Fly (Austin). 2013. PMID: 23575308 Free PMC article.
-
Derepression by depolymerization; structural insights into the regulation of Yan by Mae.Cell. 2004 Jul 23;118(2):163-73. doi: 10.1016/j.cell.2004.07.010. Cell. 2004. PMID: 15260987
-
Proteins of the ETS family with transcriptional repressor activity.Oncogene. 2000 Dec 18;19(55):6524-32. doi: 10.1038/sj.onc.1204045. Oncogene. 2000. PMID: 11175368 Review.
-
Sequence and functional properties of Ets genes in the model organism Drosophila.Oncogene. 2000 Dec 18;19(55):6409-16. doi: 10.1038/sj.onc.1204033. Oncogene. 2000. PMID: 11175357 Review.
Cited by
-
Cooperation of a polymerizing SAM domain and an intrinsically disordered region enables full SAMD1 function on chromatin.Nucleic Acids Res. 2025 Mar 20;53(6):gkaf259. doi: 10.1093/nar/gkaf259. Nucleic Acids Res. 2025. PMID: 40183636 Free PMC article.
-
Hunting for Novel Routes in Anticancer Drug Discovery: Peptides against Sam-Sam Interactions.Int J Mol Sci. 2022 Sep 8;23(18):10397. doi: 10.3390/ijms231810397. Int J Mol Sci. 2022. PMID: 36142306 Free PMC article. Review.
-
Elevated SAMD3 expression in T cells predicts improved survival in pancreatic ductal adenocarcinoma patients.Cancer Immunol Immunother. 2025 Feb 1;74(3):93. doi: 10.1007/s00262-025-03948-x. Cancer Immunol Immunother. 2025. PMID: 39891760 Free PMC article.
-
Sticky, Adaptable, and Many-sided: SAM protein versatility in normal and pathological hematopoietic states.Bioessays. 2023 Aug;45(8):e2300022. doi: 10.1002/bies.202300022. Epub 2023 Jun 15. Bioessays. 2023. PMID: 37318311 Free PMC article. Review.
-
Autoinhibition of ETV6 (TEL) DNA binding: appended helices sterically block the ETS domain.J Mol Biol. 2012 Aug 3;421(1):67-84. doi: 10.1016/j.jmb.2012.05.010. Epub 2012 May 12. J Mol Biol. 2012. PMID: 22584210 Free PMC article.
References
-
- Baker, D. A., B. Mille-Baker, S. M. Wainwright, D. Ish-Horowicz, and N. J. Dibb. 2001. Mae mediates MAP kinase phosphorylation of Ets transcription factors in Drosophila. Nature 411:330-334. - PubMed
-
- Bohlander, S. K. 2005. ETV6: a versatile player in leukemogenesis. Semin. Cancer Biol. 15:162-174. - PubMed
-
- Brunner, D., K. Ducker, N. Oellers, E. Hafen, H. Scholz, and C. Klambt. 1994. The ETS domain protein pointed-P2 is a target of MAP kinase in the sevenless signal transduction pathway. Nature 370:386-389. - PubMed
-
- Flores, G. V., H. Duan, H. Yan, R. Nagaraj, W. Fu, Y. Zou, M. Noll, and U. Banerjee. 2000. Combinatorial signaling in the specification of unique cell fates. Cell 103:75-85. - PubMed
-
- Gabay, L., H. Scholz, M. Golembo, A. Klaes, B. Z. Shilo, and C. Klambt. 1996. EGF receptor signaling induces pointed P1 transcription and inactivates Yan protein in the Drosophila embryonic ventral ectoderm. Development 122:3355-3362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
