Paralytic shellfish poisoning toxin-producing cyanobacterium Aphanizomenon gracile in northeast Germany
- PMID: 20048055
- PMCID: PMC2820976
- DOI: 10.1128/AEM.02285-09
Paralytic shellfish poisoning toxin-producing cyanobacterium Aphanizomenon gracile in northeast Germany
Abstract
Neurotoxic paralytic shellfish poisoning (PSP) toxins, anatoxin-a (ATX), and hepatotoxic cylindrospermopsin (CYN) have been detected in several lakes in northeast Germany during the last 2 decades. They are produced worldwide by members of the nostocalean genera Anabaena, Cylindrospermopsis, and Aphanizomenon. Although no additional sources of PSP toxins and ATX have been identified in German water bodies to date, the observed CYN concentrations cannot be produced solely by Aphanizomenon flos-aquae, the only known CYN producer in Germany. Therefore, we attempted to identify PSP toxin, ATX, and CYN producers by isolating and characterizing 92 Anabaena, Aphanizomenon, and Anabaenopsis strains from five lakes in northeast Germany. In a polyphasic approach, all strains were morphologically and phylogenetically classified and then tested for PSP toxins, ATX, and CYN by liquid chromatography-tandem mass spectrometry (LC-MS/MS) and enzyme-linked immunosorbent assay (ELISA) and screened for the presence of PSP toxin- and CYN-encoding gene fragments. As demonstrated by ELISA and LC-MS, 14 Aphanizomenon gracile strains from Lakes Melang and Scharmützel produced four PSP toxin variants (gonyautoxin 5 [GTX5], decarbamoylsaxitoxin [dcSTX], saxitoxin [STX], and neosaxitoxin [NEO]). GTX5 was the most prevalent PSP toxin variant among the seven strains from Lake Scharmützel, and NEO was the most prevalent among the seven strains from Lake Melang. The sxtA gene, which is part of the saxitoxin gene cluster, was found in the 14 PSP toxin-producing A. gracile strains and in 11 non-PSP toxin-producing Aphanizomenon issatschenkoi, A. flos-aquae, Anabaena planktonica, and Anabaenopsis elenkinii strains. ATX and CYN were not detected in any of the isolated strains. This study is the first confirming the role of A. gracile as a PSP toxin producer in German water bodies.
Figures
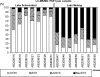


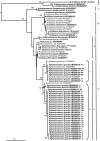
References
-
- Carmichael, W. W. 1988. Toxins of freshwater algae, p. 121-147. In A. T. Tu (ed.), Handbook of natural toxins. Volume 3. Marine toxins and venoms. Marcel Dekker, Inc., New York, NY.
-
- Carmichael, W. W. 1992. Cyanobacteria and secondary metabolites—the cyanotoxins. J. Appl. Bacteriol. 72:445-459. - PubMed
-
- Chorus, I. 2001. Cyanotoxins, occurrence, causes, consequences. Springer, Berlin, Germany.
-
- Codd, G. A., S. G. Bell, K. Kaya, C. J. Ward, K. A. Beattie, and J. S. Metcalf. 1999. Cyanobacterial toxins, exposure routes and human health. Eur. J. Phycol. 34:405-415.
-
- Dell'Aversano, C., G. K. Eaglesham, and M. A. Quilliam. 2004. Analysis of cyanobacterial toxins by hydrophilic interaction liquid chromatography-mass spectrometry. J. Chromatogr. A 1028:155-164. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

