Source of nitrous oxide emissions during the cow manure composting process as revealed by isotopomer analysis of and amoA abundance in betaproteobacterial ammonia-oxidizing bacteria
- PMID: 20048060
- PMCID: PMC2832385
- DOI: 10.1128/AEM.01394-09
Source of nitrous oxide emissions during the cow manure composting process as revealed by isotopomer analysis of and amoA abundance in betaproteobacterial ammonia-oxidizing bacteria
Abstract
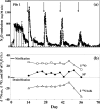
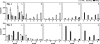
A molecular analysis of betaproteobacterial ammonia oxidizers and a N(2)O isotopomer analysis were conducted to study the sources of N(2)O emissions during the cow manure composting process. Much NO(2)(-)-N and NO(3)(-)-N and the Nitrosomonas europaea-like amoA gene were detected at the surface, especially at the top of the composting pile, suggesting that these ammonia-oxidizing bacteria (AOB) significantly contribute to the nitrification which occurs at the surface layer of compost piles. However, the (15)N site preference within the asymmetric N(2)O molecule (SP = delta(15)N(alpha) - delta(15)N(beta), where (15)N(alpha) and (15)N(beta) represent the (15)N/(14)N ratios at the center and end sites of the nitrogen atoms, respectively) indicated that the source of N(2)O emissions just after the compost was turned originated mainly from the denitrification process. Based on these results, the reduction of accumulated NO(2)(-)-N or NO(3)(-)-N after turning was identified as the main source of N(2)O emissions. The site preference and bulk delta(15)N results also indicate that the rate of N(2)O reduction was relatively low, and an increased value for the site preference indicates that the nitrification which occurred mainly in the surface layer of the pile partially contributed to N(2)O emissions between the turnings.
Figures

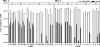
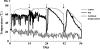
References
-
- Agriculture, Forestry and Fisheries Research Council Secretariat. 1999. Japanese feeding standard for dairy cattle. Central Association of Livestock Industry, Tokyo, Japan.
-
- Avrahami, S., W. Liesack, and R. Conrad. 2003. Effects of temperature and fertilizer on activity and community structure of soil ammonia oxidizers. Environ. Microbiol. 5:691-705. - PubMed
-
- Bothe, H., G. Jost, M. Schloter, B. B. Ward, and K. Witzel. 2000. Molecular analysis of ammonia oxidation and denitrification in natural environments. FEMS Microbiol. Rev. 24:673-690. - PubMed
-
- Bremner, J. M. 1965. Total nitrogen; inorganic forms of nitrogen, p. 1149-1255. In C. A. Black (ed.), Methods of soil analysis, part 2. Soil Science Society of America, Madison, WI.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

