Functional genetic polymorphisms in the aromatase gene CYP19 vary the response of breast cancer patients to neoadjuvant therapy with aromatase inhibitors
- PMID: 20048079
- PMCID: PMC2804935
- DOI: 10.1158/0008-5472.CAN-09-3224
Functional genetic polymorphisms in the aromatase gene CYP19 vary the response of breast cancer patients to neoadjuvant therapy with aromatase inhibitors
Abstract
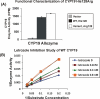
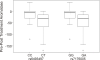
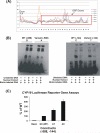
Aromatase (CYP19) is a critical enzyme in estrogen biosynthesis and aromatase inhibitors (AI) are employed widely for endocrine therapy in postmenopausal women with breast cancer. We hypothesized that single nucleotide polymorphisms (SNPs) in the CYP19 gene may alter the effectiveness of AI therapy in the neoadjuvant setting. Genomic DNA was obtained for sequencing from 52 women pre-AI and post-AI treatment in this setting. Additionally, genomic DNA obtained from 82 samples of breast cancer and 19 samples of normal breast tissue was subjected to resequencing. No differences in CYP19 sequence were observed between tumor and germ-line DNA in the same patient. A total of 48 SNPs were identified including 4 novel SNPs when compared with previous resequencing data. For genotype-phenotype association studies, we determined the levels of aromatase activity, estrone, estradiol, and tumor size in patients pre-AI and post-AI treatment. We defined two tightly linked SNPs (rs6493497 and rs7176005 in the 5'-flanking region of CYP19 exon 1.1) that were significantly associated with a greater change in aromatase activity after AI treatment. In a follow-up study of 200 women with early-stage breast cancer who were treated with adjuvant anastrozole, these same two SNPs were also associated with higher plasma estradiol levels in patients pre-AI and post-AI treatment. Electrophoretic mobility shift and reporter gene assays confirmed likely functional effects of these two SNPs on transcription of CYP19. Our findings indicate that two common genetic polymorphisms in the aromatase gene CYP19 vary the response of breast cancer patients to aromatase inhibitors.
Figures

References
-
- Geisler J, Haynes B, Anker G, Dowsett M, Lonning PE. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol. 2002;20:751–7. - PubMed
-
- Geisler J, King N, Anker G, et al. In vivo inhibition of aromatization by exemestane, a novel irreversible aromatase inhibitor, in postmenopausal breast cancer patients. Clin Cancer Res. 1998;4:2089–93. - PubMed
-
- Ingle JN, Suman VJ. Aromatase inhibitors for therapy of advanced breast cancer. J Steroid Biochem Mol Biol. 2005;95:113–9. - PubMed
-
- Ingle JN, Dowsett M, Cuzick J, Davies C. Aromatase inhibitors versus tamoxifen as adjuvant therapy for postmenopausal women with estrogen receptor positive breast cancer: meta-analyses of randomized trials of monotherapy and switching strategies. Cancer Res. 2009;69(2 Suppl S):66S.
-
- Miller WR, Dixon JM. Local endocrine effects of aromatase inhibitors within the breast. J Steroid Biochem Mol Biol. 2001;79:93–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

