(124)I-iodopyridopyrimidinone for PET of Abl kinase-expressing tumors in vivo
- PMID: 20048131
- PMCID: PMC4432838
- DOI: 10.2967/jnumed.109.066126
(124)I-iodopyridopyrimidinone for PET of Abl kinase-expressing tumors in vivo
Abstract
Because of the recent development of an iodopyridopyrimidinone Abl protein kinase inhibitor (PKI), (124)I-SKI-212230 ((124)I-SKI230), we investigated the feasibility of a PET-based molecular imaging method for the direct visualization of Abl kinase expression and PKI treatment.
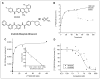
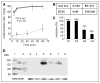
Methods: In vitro pharmacokinetic properties, including specific and nonspecific binding of (124)I-SKI230 to its Abl kinase target and interaction with other PKIs, were assessed in cell-free medium and chronic myelogenous leukemia (CML) cells overexpressing BCR-Abl (K562), in comparison with BT-474 cells that are low in Abl expression. In a xenograft tumor model, we assessed the in vivo pharmacokinetics of (124)I-SKI230 using PET and postmortem tissue sampling. We also tested a paradigm of (124)I-SKI230 PET after treatment of the animal with a dose of Abl-specific PKI for the monitoring of the tumor response.
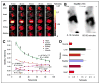
Results: In vitro studies confirmed that SKI230 binds to Abl kinase with nanomolar affinity, that selective uptake occurs in cell lines known to express Abl kinase, that RNAi knock-down supports specificity of cellular uptake due to Abl kinase, and that imatinib, an archetype Abl PKI, completely displaces SKI230. With SKI230, we obtained successful in vivo PET of Abl-expressing human tumors in a nude rat. We were also able to demonstrate evidence of substrate inhibition of in vivo radiotracer uptake in the xenograft tumor after treatment of the animal as a model of PKI treatment monitoring.
Conclusion: These results support the hypothesis that molecular imaging using PET will be useful for the study of in vivo pharmacodynamics of Abl PKI molecular therapy in humans.
Figures

Similar articles
-
SKI-606, a 4-anilino-3-quinolinecarbonitrile dual inhibitor of Src and Abl kinases, is a potent antiproliferative agent against chronic myelogenous leukemia cells in culture and causes regression of K562 xenografts in nude mice.Cancer Res. 2003 Jan 15;63(2):375-81. Cancer Res. 2003. PMID: 12543790
-
Synthesis and in vitro examination of [124I]-, [125I]- and [131I]-2-(4-iodophenylamino) pyrido[2,3-d]pyrimidin-7-one radiolabeled Abl kinase inhibitors.Nucl Med Biol. 2005 May;32(4):313-21. doi: 10.1016/j.nucmedbio.2005.01.008. Nucl Med Biol. 2005. PMID: 15878500
-
Inhibition of Bcr-Abl kinase activity by PD180970 blocks constitutive activation of Stat5 and growth of CML cells.Oncogene. 2002 Dec 12;21(57):8804-16. doi: 10.1038/sj.onc.1206028. Oncogene. 2002. PMID: 12483533
-
Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia.Clin Ther. 2007 Nov;29(11):2289-308. doi: 10.1016/j.clinthera.2007.11.005. Clin Ther. 2007. PMID: 18158072 Review.
-
[Tyrosine kinase inhibitor as a therapeutic drug for chronic myelogenous leukemia and gastrointestinal stromal tumor].Nihon Yakurigaku Zasshi. 2003 Dec;122(6):482-90. doi: 10.1254/fpj.122.482. Nihon Yakurigaku Zasshi. 2003. PMID: 14639002 Review. Japanese.
Cited by
-
Recent Advances in the Development and Application of Radiolabeled Kinase Inhibitors for PET Imaging.Molecules. 2015 Dec 9;20(12):22000-27. doi: 10.3390/molecules201219816. Molecules. 2015. PMID: 26690113 Free PMC article. Review.
-
C2c: turning cancer into chronic disease.Genome Med. 2014 May 28;6(5):38. doi: 10.1186/gm555. eCollection 2014. Genome Med. 2014. PMID: 24944585 Free PMC article. No abstract available.
-
Novel Receptor Tyrosine Kinase Pathway Inhibitors for Targeted Radionuclide Therapy of Glioblastoma.Pharmaceuticals (Basel). 2021 Jun 29;14(7):626. doi: 10.3390/ph14070626. Pharmaceuticals (Basel). 2021. PMID: 34209513 Free PMC article. Review.
-
Radiolabeled small molecule protein kinase inhibitors for imaging with PET or SPECT.Molecules. 2010 Nov 15;15(11):8260-78. doi: 10.3390/molecules15118260. Molecules. 2010. PMID: 21079565 Free PMC article. Review.
-
Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo.Nat Commun. 2013;4:1504. doi: 10.1038/ncomms2506. Nat Commun. 2013. PMID: 23422672 Free PMC article.
References
-
- Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem Cells. 1998;16:413–428. - PubMed
-
- Galimberti S, Cervetti G, Guerrini F, et al. Quantitative molecular monitoring of BCR-ABL and MDR1 transcripts in patients with chronic myeloid leukemia during imatinib treatment. Cancer Genet Cytogenet. 2005;162:57–62. - PubMed
-
- Shu HK, Pelley RJ, Kung HJ. Tissue-specific transformation by epidermal growth factor receptor: a single point mutation within the ATP-binding pocket of the erbB product increases its intrinsic kinase activity and activates its sarcomagenic potential. Proc Natl Acad Sci U S A. 1990 Dec;87:9103–9107. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
