Viral induction of the zinc finger antiviral protein is IRF3-dependent but NF-kappaB-independent
- PMID: 20048147
- PMCID: PMC2825402
- DOI: 10.1074/jbc.M109.054486
Viral induction of the zinc finger antiviral protein is IRF3-dependent but NF-kappaB-independent
Abstract
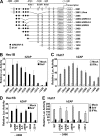
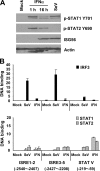
The zinc finger antiviral protein (ZAP) is an interferon-stimulated gene that restricts the replication of retroviruses, alphaviruses, and filoviruses. Relatively little is known, however, regarding the detailed mechanism of ZAP induction during viral infections. We show that, although being inducible by either interferon or virus, expression of ZAP is more efficiently activated by virus than are several other classical interferon-stimulated genes and that viral induction of ZAP occurs under the direct control of interferon regulatory factor 3 (IRF3) independent of interferon paracrine/autocrine signaling. ZAP was up-regulated in cells unresponsive to type I and III interferons upon engagement of TLR3, retinoic inducible gene I/melanoma differentiation-associated gene 5 pathways, or ectopic expression of a constitutively active IRF3 mutant. Conversely, induction of ZAP by virus or dsRNA was severely impaired in cells expressing a dominant-negative mutant IRF3 and completely abrogated in cells lacking IRF3. In contrast to IRF3, ZAP induction was independent of NF-kappaB activity. Mutational analysis of the human ZAP promoter revealed that multiple interferon-stimulated response elements far distal to the transcription start site serve redundantly to control IRF3-dependent induction of ZAP transcription. Chromatin immunoprecipitation assays demonstrated that IRF3 selectively binds the distal interferon-stimulated response elements in human ZAP promoter following viral infection. Collectively, these data suggest that ZAP is a direct target gene of IRF3 action in cellular antiviral responses.
Figures


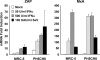



References
-
- Sen G. C. (2001) Annu. Rev. Microbiol. 55, 255–281 - PubMed
-
- Müller U., Steinhoff U., Reis L. F., Hemmi S., Pavlovic J., Zinkernagel R. M., Aguet M. (1994) Science 264, 1918–1921 - PubMed
-
- Hiscott J. (2007) J. Biol. Chem. 282, 15325–15329 - PubMed
-
- Osterlund P. I., Pietilä T. E., Veckman V., Kotenko S. V., Julkunen I. (2007) J. Immunol. 179, 3434–3442 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA140346/CA/NCI NIH HHS/United States
- U19-AI066316/AI/NIAID NIH HHS/United States
- R21-AI081058/AI/NIAID NIH HHS/United States
- R01 AI069285/AI/NIAID NIH HHS/United States
- U19 AI040035/AI/NIAID NIH HHS/United States
- R01 CA133322/CA/NCI NIH HHS/United States
- R01-AI069285/AI/NIAID NIH HHS/United States
- R21-DA018054/DA/NIDA NIH HHS/United States
- U19-AI40035/AI/NIAID NIH HHS/United States
- R21 AI081058/AI/NIAID NIH HHS/United States
- R21 DA018054/DA/NIDA NIH HHS/United States
- U19 AI066316/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources

