Rheb GTPase controls apoptosis by regulating interaction of FKBP38 with Bcl-2 and Bcl-XL
- PMID: 20048149
- PMCID: PMC2838284
- DOI: 10.1074/jbc.M109.092353
Rheb GTPase controls apoptosis by regulating interaction of FKBP38 with Bcl-2 and Bcl-XL
Abstract
FKBP38 is a member of the family of FK506-binding proteins that acts as an inhibitor of the mammalian target of rapamycin (mTOR). The inhibitory action of FKBP38 is antagonized by Rheb, an oncogenic small GTPase, which interacts with FKBP38 and prevents its association with mTOR. In addition to the role in mTOR regulation, FKBP38 is also involved in binding and recruiting Bcl-2 and Bcl-X(L), two anti-apoptotic proteins, to mitochondria. In this study, we investigated the possibility that Rheb controls apoptosis by regulating the interaction of FKBP38 with Bcl-2 and Bcl-X(L). We demonstrate in vitro that the interaction of FKBP38 with Bcl-2 is regulated by Rheb in a GTP-dependent manner. In cultured cells, the interaction is controlled by Rheb in response to changes in amino acid and growth factor conditions. Importantly, we found that the Rheb-dependent release of Bcl-X(L) from FKBP38 facilitates the association of this anti-apoptotic protein with the pro-apoptotic protein Bak. Consequently, when Rheb activity increases, cells become more resistant to apoptotic inducers. Our findings reveal a novel mechanism through which growth factors and amino acids control apoptosis.
Figures
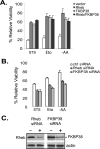

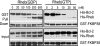

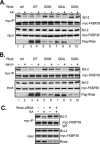
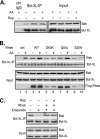
References
-
- Crino P. B., Nathanson K. L., Henske E. P. (2006) N. Engl. J. Med. 355, 1345–1356 - PubMed
-
- Mak B. C., Yeung R. S. (2004) Cancer Invest. 22, 588–603 - PubMed
-
- Li Y., Corradetti M. N., Inoki K., Guan K. L. (2004) Trends Biochem. Sci. 29, 32–38 - PubMed
-
- Manning B. D., Cantley L. C. (2003) Trends Biochem. Sci. 28, 573–576 - PubMed
-
- Inoki K., Corradetti M. N., Guan K. L. (2005) Nat. Genet. 37, 19–24 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

