Identification of a key residue mediating bone morphogenetic protein (BMP)-6 resistance to noggin inhibition allows for engineered BMPs with superior agonist activity
- PMID: 20048150
- PMCID: PMC2852956
- DOI: 10.1074/jbc.M109.087197
Identification of a key residue mediating bone morphogenetic protein (BMP)-6 resistance to noggin inhibition allows for engineered BMPs with superior agonist activity
Abstract
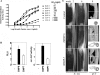
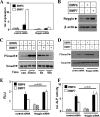
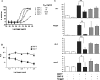
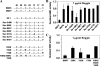
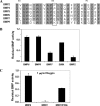
Bone morphogenetic proteins (BMPs) are used clinically to induce new bone formation in spinal fusions and long bone non-union fractures. However, large amounts of BMPs are needed to achieve these effects. BMPs were found to increase the expression of antagonists, which potentially limit their therapeutic efficacy. However, the relative susceptibility of osteoinductive BMPs to different antagonists is not well characterized. Here we show that BMP-6 is more resistant to noggin inhibition and more potent in promoting osteoblast differentiation in vitro and inducing bone regeneration in vivo when compared with its closely related BMP-7 paralog. Noggin was found to play a critical role as a negative feedback regulator of BMP-7 but not BMP-6-induced biological responses. Using BMP-6/7 chimeras, we identified lysine 60 as a key residue conferring noggin resistance within the BMP-6 protein. A remarkable correlation was found between the presence of a lysine at this position and noggin resistance among a panel of osteoinductive BMPs. Introduction of a lysine residue at the corresponding positions of BMP-2 and BMP-7 allowed for molecular engineering of recombinant BMPs with increased resistance to noggin antagonism.
Figures


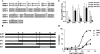

Similar articles
-
RNA interference for noggin enhances the biological activity of bone morphogenetic proteins in vivo and in vitro.J Bone Miner Metab. 2009;27(4):402-11. doi: 10.1007/s00774-009-0054-x. Epub 2009 Feb 28. J Bone Miner Metab. 2009. PMID: 19252814
-
Biphasic effects of transforming growth factor β on bone morphogenetic protein-induced osteoblast differentiation.J Bone Miner Res. 2011 Jun;26(6):1178-87. doi: 10.1002/jbmr.313. J Bone Miner Res. 2011. PMID: 21611961
-
The role of noggin in human mesenchymal stem cell differentiation.J Cell Biochem. 2007 Mar 1;100(4):824-34. doi: 10.1002/jcb.21132. J Cell Biochem. 2007. PMID: 17133353
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
Application of Cytokines of the Bone Morphogenetic Protein (BMP) Family in Spinal Fusion - Effects on the Bone, Intervertebral Disc and Mesenchymal Stromal Cells.Curr Stem Cell Res Ther. 2019;14(8):618-643. doi: 10.2174/1574888X14666190628103528. Curr Stem Cell Res Ther. 2019. PMID: 31455201 Free PMC article. Review.
Cited by
-
BMP2 and mechanical loading cooperatively regulate immediate early signalling events in the BMP pathway.BMC Biol. 2012 Apr 30;10:37. doi: 10.1186/1741-7007-10-37. BMC Biol. 2012. PMID: 22540193 Free PMC article.
-
The Role Of BMPs in the Regulation of Osteoclasts Resorption and Bone Remodeling: From Experimental Models to Clinical Applications.Front Immunol. 2022 Apr 26;13:869422. doi: 10.3389/fimmu.2022.869422. eCollection 2022. Front Immunol. 2022. PMID: 35558080 Free PMC article. Review.
-
A novel autologous bone graft substitute containing rhBMP6 in autologous blood coagulum with synthetic ceramics for reconstruction of a large humerus segmental gunshot defect in a dog: The first veterinary patient to receive a novel osteoinductive therapy.Bone Rep. 2021 Feb 25;14:100759. doi: 10.1016/j.bonr.2021.100759. eCollection 2021 Jun. Bone Rep. 2021. PMID: 33732816 Free PMC article.
-
Cancer-associated fibroblast-derived Gremlin 1 promotes breast cancer progression.Breast Cancer Res. 2019 Sep 18;21(1):109. doi: 10.1186/s13058-019-1194-0. Breast Cancer Res. 2019. PMID: 31533776 Free PMC article.
-
Time to consider fracture nonunion an orphan disease? An update into pathophysiology, epidemiology and therapeutic solutions.Eur J Trauma Emerg Surg. 2025 Jul 21;51(1):255. doi: 10.1007/s00068-025-02918-3. Eur J Trauma Emerg Surg. 2025. PMID: 40691747 Free PMC article. Review.
References
-
- Urist M. R. (1965) Science 150, 893–899 - PubMed
-
- Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., Wang E. A. (1988) Science 242, 1528–1534 - PubMed
-
- Reddi A. H., Reddi A. (2009) Cytokine Growth Factor Rev. 20, 341–342 - PubMed
-
- Miyazono K., Maeda S., Imamura T. (2005) Cytokine Growth Factor Rev. 16, 251–263 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

