G protein betagamma subunits regulate cell adhesion through Rap1a and its effector Radil
- PMID: 20048162
- PMCID: PMC2825450
- DOI: 10.1074/jbc.M109.069948
G protein betagamma subunits regulate cell adhesion through Rap1a and its effector Radil
Abstract
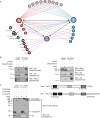
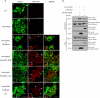
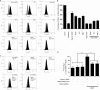
The activation of several G protein-coupled receptors is known to regulate the adhesive properties of cells in different contexts. Here, we reveal that Gbetagamma subunits of heterotrimeric G proteins regulate cell-matrix adhesiveness by activating Rap1a-dependent inside-out signals and integrin activation. We show that Gbetagamma subunits enter in a protein complex with activated Rap1a and its effector Radil and establish that this complex is required downstream of receptor stimulation for the activation of integrins and the positive modulation of cell-matrix adhesiveness. Moreover, we demonstrate that Gbetagamma and activated Rap1a promote the translocation of Radil to the plasma membrane at sites of cell-matrix contacts. These results add to the molecular understanding of how G protein-coupled receptors impinge on cell adhesion and suggest that the Gbetagamma x Rap1 x Radil complex plays important roles in this process.
Figures

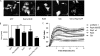



References
-
- Doitsidou M., Reichman-Fried M., Stebler J., Köprunner M., Dörries J., Meyer D., Esguerra C. V., Leung T., Raz E. (2002) Cell 111, 647–659 - PubMed
-
- Scott I. C., Masri B., D'Amico L. A., Jin S. W., Jungblut B., Wehman A. M., Baier H., Audigier Y., Stainier D. Y. (2007) Dev. Cell 12, 403–413 - PubMed
-
- Carmona G., Chavakis E., Koehl U., Zeiher A. M., Dimmeler S. (2008) Blood 111, 2640–2646 - PubMed
-
- Höpken U. E., Lu B., Gerard N. P., Gerard C. (1996) Nature 383, 86–89 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

