Src-induced tyrosine phosphorylation of VE-cadherin is not sufficient to decrease barrier function of endothelial monolayers
- PMID: 20048167
- PMCID: PMC2844154
- DOI: 10.1074/jbc.M109.079277
Src-induced tyrosine phosphorylation of VE-cadherin is not sufficient to decrease barrier function of endothelial monolayers
Abstract
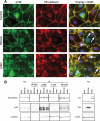
Activation of Src family kinases (SFK) and the subsequent phosphorylation of VE-cadherin have been proposed as major regulatory steps leading to increases in vascular permeability in response to inflammatory mediators and growth factors. To investigate Src signaling in the absence of parallel signaling pathways initiated by growth factors or inflammatory mediators, we activated Src and SFKs by expression of dominant negative Csk, expression of constitutively active Src, or knockdown of Csk. Activation of SFK by overexpression of dominant negative Csk induced VE-cadherin phosphorylation at tyrosines 658, 685, and 731. However, dominant negative Csk expression was unable to induce changes in the monolayer permeability. In contrast, expression of constitutively active Src decreased barrier function and promoted VE-cadherin phosphorylation on tyrosines 658 and 731, although the increase in VE-cadherin phosphorylation preceded the increase in permeability by 4-6 h. Csk knockdown induced VE-cadherin phosphorylation at sites 658 and 731 but did not induce a loss in barrier function. Co-immunoprecipitation and immunofluorescence studies suggest that phosphorylation of those sites did not impair VE-cadherin ability to bind p120 and beta-catenin or the ability of these proteins to localize at the plasma membrane. Taken together, our data show that Src-induced tyrosine phosphorylation of VE-cadherin is not sufficient to promote an increase in endothelial cell monolayer permeability and suggest that signaling leading to changes in vascular permeability in response to inflammatory mediators or growth factors may require VE-cadherin tyrosine phosphorylation concurrently with other signaling pathways to promote loss of barrier function.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

