Heterozygous deficiency of delta-catenin impairs pathological angiogenesis
- PMID: 20048286
- PMCID: PMC2812534
- DOI: 10.1084/jem.20091097
Heterozygous deficiency of delta-catenin impairs pathological angiogenesis
Erratum in
- J Exp Med. 2010 Feb 15;207(2):443
- J Exp Med. 2010 Mar 15;207(3):669
Abstract
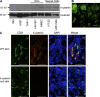
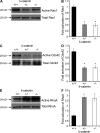
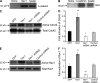
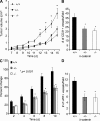
Vascular and neuronal networks share a similar branching morphology, and emerging evidence implicates common mechanisms in the formation of both systems. delta-Catenin is considered a neuronal catenin regulating neuron cell-cell adhesion and cell motility. Here, we report expression of delta-catenin in vascular endothelium, and show that deletion of only one allele of delta-catenin is sufficient to impair endothelial cell motility and vascular assembly in vitro and pathological angiogenesis in vivo, thereby inhibiting tumor growth and wound healing. In contrast, deletion of one or both allele of delta-catenin had no effects on hormone-induced physiological angiogenesis in the uterus. Molecular analysis confirmed a gene dosage effect of delta-catenin on Rho GTPase activity. Moreover, we show that inflammatory cytokines, but not angiogenic factors, regulate delta-catenin expression, and the levels of delta-catenin positively correlate to human lung cancers. Collectively, our data suggest that inflammation, commonly associated with disease conditions, induces delta-catenin expression that specifically regulates pathological, and not physiological, angiogenesis. Because only pathological angiogenesis is sensitive to decreased levels of delta-catenin, this may provide a good target for antiangiogenic therapy.
Figures


References
-
- Grosheva I., Shtutman M., Elbaum M., Bershadsky A.D. 2001. p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell-cell contact formation and regulation of cell locomotion. J. Cell Sci. 114:695–707 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

