Humanized SFTPA1 and SFTPA2 transgenic mice reveal functional divergence of SP-A1 and SP-A2: formation of tubular myelin in vivo requires both gene products
- PMID: 20048345
- PMCID: PMC2852938
- DOI: 10.1074/jbc.M109.046243
Humanized SFTPA1 and SFTPA2 transgenic mice reveal functional divergence of SP-A1 and SP-A2: formation of tubular myelin in vivo requires both gene products
Abstract
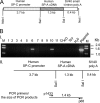
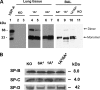
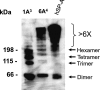
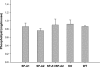
Surfactant protein A (SP-A) plays a role in lung innate immunity and surfactant-related functions. Two functional genes, SP-A1 (SFTPA1) and SP-A2 (SFTPA2), are present in humans and primates (rodents have one gene). Single gene SP-A1 or SP-A2 proteins expressed in vitro are functional. To study their role in vivo, we generated humanized transgenic (hTG) C57BL/6 mice, SP-A1(6A(4)) and SP-A2(1A(3)). The SP-A cDNA in experimental constructs was driven by the 3.7-kb SP-C promoter. Positive hTG mice were bred with SP-A knock-out mice to generate F8 offspring for study. Epithelial alveolar type II cells were SP-A-positive, and Clara cells were negative by immunohistochemistry in hTG mice. The levels of SP-A in lungs of two hTG lines used were comparable with those in human lungs. Southern blot analysis indicated that two cDNA copies of either SP-A1(6A(4)) or SP-A2(1A(3)) were integrated as a concatemer into the genome of each of the two hTG lines. Electron microscopy analysis revealed that hTG mice with a single SP-A1(6A(4)) or SP-A2(1A(3)) gene product lacked tubular myelin (TM), but hTG mice carrying both had TM. Furthermore, TM was observed in human bronchoalveolar lavage fluid only if both SP-A1 and SP-A2 gene products were present and not in those containing primarily (>99.7%) either SP-A1 or SP-A2 gene products. In vivo rescue study confirmed that TM can only be restored after administering exogenous SP-A containing both SP-A1 and SP-A2 into the lungs of SP-A knock-out mice. These observations indicate that SP-A1 and SP-A2 diverged functionally at least in terms of TM formation.
Figures





Similar articles
-
Differential effects of innate immune variants of surfactant protein-A1 (SFTPA1) and SP-A2 (SFTPA2) in airway function after Klebsiella pneumoniae infection and sex differences.Respir Res. 2018 Feb 3;19(1):23. doi: 10.1186/s12931-018-0723-1. Respir Res. 2018. PMID: 29394894 Free PMC article.
-
Survival of Surfactant Protein-A1 and SP-A2 Transgenic Mice After Klebsiella pneumoniae Infection, Exhibits Sex-, Gene-, and Variant Specific Differences; Treatment With Surfactant Protein Improves Survival.Front Immunol. 2018 Oct 16;9:2404. doi: 10.3389/fimmu.2018.02404. eCollection 2018. Front Immunol. 2018. PMID: 30459763 Free PMC article.
-
Differential Effects of Human SP-A1 and SP-A2 on the BAL Proteome and Signaling Pathways in Response to Klebsiella pneumoniae and Ozone Exposure.Front Immunol. 2019 Mar 26;10:561. doi: 10.3389/fimmu.2019.00561. eCollection 2019. Front Immunol. 2019. PMID: 30972061 Free PMC article.
-
Human Surfactant Protein SP-A1 and SP-A2 Variants Differentially Affect the Alveolar Microenvironment, Surfactant Structure, Regulation and Function of the Alveolar Macrophage, and Animal and Human Survival Under Various Conditions.Front Immunol. 2021 Aug 17;12:681639. doi: 10.3389/fimmu.2021.681639. eCollection 2021. Front Immunol. 2021. PMID: 34484180 Free PMC article. Review.
-
Genetic complexity of the human innate host defense molecules, surfactant protein A1 (SP-A1) and SP-A2--impact on function.Crit Rev Eukaryot Gene Expr. 2009;19(2):125-37. doi: 10.1615/critreveukargeneexpr.v19.i2.30. Crit Rev Eukaryot Gene Expr. 2009. PMID: 19392648 Free PMC article. Review.
Cited by
-
Genetic complexity of the human surfactant-associated proteins SP-A1 and SP-A2.Gene. 2013 Dec 1;531(2):126-32. doi: 10.1016/j.gene.2012.09.111. Epub 2012 Oct 12. Gene. 2013. PMID: 23069847 Free PMC article. Review.
-
Sex and SP-A2 Dependent NAD(H) Redox Alterations in Mouse Alveolar Macrophages in Response to Ozone Exposure: Potential Implications for COVID-19.Antioxidants (Basel). 2020 Sep 25;9(10):915. doi: 10.3390/antiox9100915. Antioxidants (Basel). 2020. PMID: 32992843 Free PMC article.
-
Sex differences in the response of the alveolar macrophage proteome to treatment with exogenous surfactant protein-A.Proteome Sci. 2012 Jul 23;10(1):44. doi: 10.1186/1477-5956-10-44. Proteome Sci. 2012. PMID: 22824420 Free PMC article.
-
The Lung Alveolar Cell (LAC) miRNome and Gene Expression Profile of the SP-A-KO Mice After Infection With and Without Rescue With Human Surfactant Protein-A2 (1A0).Front Immunol. 2022 Jul 1;13:854434. doi: 10.3389/fimmu.2022.854434. eCollection 2022. Front Immunol. 2022. PMID: 35844510 Free PMC article.
-
Differences in the alveolar macrophage proteome in transgenic mice expressing human SP-A1 and SP-A2.J Proteom Genom Res. 2013 Apr 1;1(2):2-26. doi: 10.14302/issn.2326-0793.jpgr-12-207. J Proteom Genom Res. 2013. PMID: 24729982 Free PMC article.
References
-
- Wright J. R. (2005) Nat. Rev. Immunol. 5, 58–68 - PubMed
-
- Bates S. R., Dodia C., Tao J. Q., Fisher A. B. (2008) Am. J. Physiol. Lung Cell Mol. Physiol. 294, L325–L333 - PubMed
-
- Ikegami M., Elhalwagi B. M., Palaniyar N., Dienger K., Korfhagen T., Whitsett J. A., McCormack F. X. (2001) J. Biol. Chem. 276, 38542–38548 - PubMed
-
- Jain D., Dodia C., Bates S. R., Hawgood S., Poulain F. R., Fisher A. B. (2003) Am. J. Physiol. Lung Cell Mol. Physiol. 284, L759–L765 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

